Abstract
Background
The role of preoperative chemotherapy for breast cancer is evolving. We initiated a prospective trial of sequential preoperative paclitaxel and doxorubicin-based combination chemotherapy in patients with stage I (tumor>1 cm), II, or IIIA disease and evaluated its effect on breast-conservation therapy (BCT) eligibility.
Methods
Pathology findings for the initial 100 consecutive patients who underwent surgery were analyzed.
Results
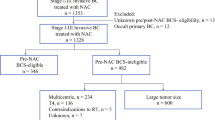
The median tumor size at presentation was 2.4 cm, and 39% of patients were deemed eligible for BCT. After chemotherapy, the median tumor size decreased to 1.0 cm (P<.001), and 59% of patients seemed BCT eligible (BCT conversion rate 34% among patients initially assessed as BCT ineligible;P<.001). Final pathology confirmed BCT feasibility in 90% of patients assessed as BCT candidates before surgery. The pathology from mastectomy specimens revealed BCT feasibility in 11 (27%) of 41 patients deemed BCT ineligible. Multivariate analysis revealed lobular histology, multicentricity, and calcifications, but not age, initial tumor size, or nodal status to predict final pathology indicating BCT ineligibility.
Conclusions
Induction chemotherapy improves BCT eligibility for breast cancer patients. Improved breast imaging methods after chemotherapy are necessary to improve accuracy in predicting the feasibility of BCT, especially in patients presenting with diffuse calcifications or multicentricity.
Similar content being viewed by others
References
Hunt KK, Ames FC, Singletary SE, Buzdar AU, Hortobagyi GN. Locally advanced noninflammatory breast cancer.Surg Clin North Am 1996;76:393–410.
Fisher B, Brown A, Mamounas E et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from the National Surgical Adjuvant Breast and Bowel Project B-18.J Clin Oncol 1997;15:2483–93.
Fisher B, Wolmark N, Mamounas E, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer.J Clin Oncol 1998;16:2672–85.
Hortobagyi GN, Singletary SE, McNeese MD. Treatment of locally advanced and inflammatory breast cancer. In: Harris JR, Lippman ME, Morrow M, Hellman S, eds.Diseases of the Breast. Philadelphia: Lippincott-Raven, 1996:585–99.
Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer.J Clin Oncol 2000;18:1558–69.
McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer.Arch Surg 1989;124:21–5.
Broadwater JR, Edwards MJ, Kuglen C, Hortobagyi GN, Ames FC, Balch CM. Mastectomy following preoperative chemotherapy.Ann Surg 1991;213:126–9.
Singletary SE, McNeese MD, Hortobagyi GN. Feasibility of breast conservation surgery after induction chemotherapy for locally advanced breast cancer.Cancer 1992;69:2849–52.
Peoples G, Heaton K, Booser D, et al. Breast conservation therapy for large primary and locally advanced breast cancers after induction chemotherapy (abstract No. 25). Society of Surgical Oncology 51st Annual Cancer Symposium, San Diego, CA, March 26–29, 1998.
Bonnadonna G, Veronesi U, Brambilla C, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more.J Natl Cancer Inst 1990;82:1539–45.
Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm.Ann Oncol 1991;2:347–54.
Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up.Ann Oncol 1999;10:47–52.
Semiglazov VF, Topuzov EE, Bavli JL, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIB-IIIA breast cancer.Ann Oncol 1994; 5:591–5.
Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumors considered too large for breast conserving surgery: preliminary results of a randomised trial—S6.Eur J Cancer 1994;30A:645–52.
Scholl SM, Pierga JY, Asselain B, et al. Breast tumour response to primary chemotherapy predicts local and distant control as well as survival.Eur J Cancer 1995;31A:1969–75.
Powles TJ, Hickish TF, Makris A, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer.J Clin Oncol 1995;13:547–52.
Makris A, Powles TJ, Ashley SE, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer.Ann Oncol 1998; 9:1179–84.
Kuerer HM, Newman LA, Smith TL et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy.J Clin Oncol 1999;17:460–9.
Buzdar AU, Singletary SE, Theriault RL, et al. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer.J Clin Oncol 1999;17:3412–7.
Davidson NG, Chick JB, Perren TJ, Campbell N, Thompson JM, Chan YT. A phase II study of single agent paclitaxel in patients at first relapse following initial treatment for breast cancer.Clin Oncol 1996;8:358–62.
Seidman AD. One-hour paclitaxel via weekly infusion: dose-intensity with enhanced therapeutic index.Oncology 1998;12:19–22.
Winchester DP, Cox JD. Standards for diagnosis and management of invasive breast carcinoma.CA Cancer J Clin 1998;48:83–107.
Abraham DC, Jones RC, Jones SE, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging.Cancer 1996;78:91–100.
Avril N, Rose CA, Schelling M, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations.J Clin Oncol 2000;18:3495–502.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newman, L.A., Buzdar, A.U., Singletary, S.E. et al. A prospective trial of preoperative chemotherapy in resectable breast cancer: Predictors of breast-conservation therapy feasibility. Annals of Surgical Oncology 9, 228–234 (2002). https://doi.org/10.1007/BF02573059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02573059