Summary
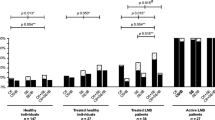
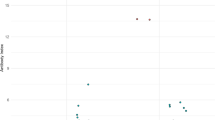
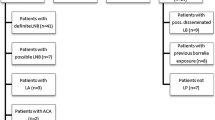
The sensitivity and specificity of three confirmatory assays for the serodiagnosis of neuroborreliosis were investigated. Samples from 96 patients with proven neuroborreliosis, 80 healthy volunteers, 20 patients with neurosyphilis and 20 patients with recent infections with Epstein-Barr virus (EBV) were tested for borrelial antibodies by immunoblotting,Borrelia burgdorferi sensu lato sonicate EIA following pre-absorption of cross-reactive antibodies (Abs-EIA) and by a so-called RECO-EIA using the following recombinant borrelial proteins as antigens: a 14 kDa-internal flagellin fragment, the outer surface protein C (23 kDa) and the high molecular mass protein p83 (83 kDa). the immunoblots were evaluated according to the criteria published byEngström et al. andHauser et al. An evaluation of IgM and/or IgG antibodies revealed a considerably higher sensitivity for the RECO-EIA (94%) compared to the Abs-EIA (82%, P<0.0001). Evaluation of the immunoblot according to the criteria ofHauser was significantly more sensitive than according to the criteria ofEngström (89 vs 51%, P=0.0003). A higher sensitivity was demonstrated for IgM (54 vs 22%) and IgG antibodies (64 vs 24%). When both findings from RECO-EIA and immunoblotting were considered, positive findings in the first step assay (sonicate EIA without pre-absorption) were confirmed in 97% of patients. When samples were tested for IgM antibodies, the specificities of the three confirmatory assays did not differ significantly, but in the case of IgG antibodies, the immunoblot (Hauser: P=0.013;Engström: P=0.004) and the RECO-EIA (P=0.02), were more specific than the Abs-EIA. It is concluded that the immunoblot (evaluated according toHauser) and the RECO-EIA are both suitable as confirmatory assays in the serological diagnosis of neuroborreliosis. Monoclonal antibodies are mandatory tools in the evaluation of the immunoblot.
Similar content being viewed by others
References
Tilton, R. C.: Laboratory aids for the diagnosis ofBorrelia burgdorferi infection. Journal of Spirochetal and Tick-Borne Diseases 1 (1994) 18–23.
CDC/DVBID: Standardisation of Lyme disease serodiagnosis: working groups meet to chart progress. CDC 5 (1994) 1–3.
Burkert, S., Rossler, D., Munchhoff, P., Wilske, B.: Development of enzyme-linked immunosorbent assays using recombinant borrelial antigens for serodiagnosis ofBorrelia burgdorferi infection. Med. Microbiol. Immunol. (Berlin) 185 (1996) 49–57.
Magnarelli, L. A., Fikrig, E., Padula, S. J., Anderson, J. F., Flavell, R. A.: Use of recombinant antigens ofBorrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J. Clin. Microbiol. 34 (1996) 237–240.
Mathiesen, M. J., Hansen, K., Axelsen, N., Halkier-Sorensen, L., Theisen, M.: Analysis of the human antibody response to outer surface protein C (OspC ofBorrelia burgdorferi sensu stricto, B. garinii, andB. afzelii. Med. Microbiol. Immunol. (Berlin) 185 (1996) 121–129.
Rasiah, C., Schiltz, E., Reichert, J., Vogt, A.: Purification and characterization of a tryptic peptide ofBorrelia burgdorferi flagellin, which reduces cross-reactivity in immunoblots and ELISA. J. Gen. Microbiol. 138 (1992), 147–154.
Rauer, S., Kayser M., Neubert, U., Rasiah, C., Vogt, A.: Establishment of enzyme-linked immunosorbent assay using purified recombinant 83-kilodalton antigen ofBorrelia burgdorferi sensu stricto andBorrelia afzelii for serodiagnosis of Lyme disease. J. Clin. Microbiol. 33 (1995) 2596–2600.
Hauser, U., Lehnert, G., Lobentanzer, R., Wilske, B.: Interpretation criteria for standardized Western blots for three European species ofBorrelia burgdorferi sensu lato. J. Clin. Microbiol. 35 (1997) 1433–1444.
Engström, S. M., Shoop, E., Johnson, R.C.: Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33 (1995) 419–427.
Kaiser, R.: Variable CSF findings in early and late Lyme neuroborreliosis: a follow-up study in 47 patients. J. Neurol. 242 (1994) 26–36.
Kaiser, R., Lucking, C. H.: Intrathecal synthesis of specific antibodies in neuroborreliosis. Comparison of different ELISA techniques and calculation methods. J. Neurol. Sci. 118 (1993) 64–72.
Müller, F., Moskophidis, M., Borkhard, H. L.: Detection of immunoglobulin M antibodies toTreponema pallidum in a modified immunosorbent assay. Eur. J. Clin. Microbiol. 6 (1987) 35–39.
Kaiser, R., Kern, A., Kampa, D., Neumann-Haeflin, D.: Prevalence of antibodies toBorrelia burgdorferi and tick-borne encephalitis virus in an endemic region in southern Germany. Zentralbl. Bakteriol. 286 (1997) 534–541.
Fingerle, V., Hauser, U., Liegl, G., Petko, B., Preac-Mursic, V., Wilske, B.: Expression of outer surface proteins A and C ofBorrelia burgdorferi inIxodes ricinus. J. Clin. Microbiol. 33 (1995) 1867–1869.
Fawcett, P. T., O'Brien, A.E., Doughty, R.A.: An adsorption procedure to increase the specificity of enzyme-linked immunosorbent assays for Lyme disease without decreasing sensitivity. Arthritis Rheum. 32 (1989) 1041–1044.
Magnarelli, L. A., Miller, J. N., Anderson, J. F., Riviere, G. R.: Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J. Clin. Microbiol. 28 (1990) 1276–1279.
Wallich, R., Brenner, C., Kramer, M. D., Simon, M. M.: Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, ofBorrelia burgdorferi expressed onlyin vivo. Infect. Immun. 63 (1995) 3327–3335.
Norris, S. J., Carter, C., Howell, J. K., Barbour, A. G.: Low-passage-associated proteins ofBorrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60 (1992) 4662–4672.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaiser, R., Rauer, S. Serodiagnosis of neuroborreliosis: Comparison of reliability of three confirmatory assays. Infection 27, 177–182 (1999). https://doi.org/10.1007/BF02561524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02561524