Abstract
The heat effects of the reactions of formation of ethylenediamine-copper(II) complexes were determined calorimetrically in mixtures of water with ethanol, acetone and dimethylsulfoxide. The results were interpreted in terms of the enthalpies of transfer (Δt H 0) of the complex former, the ligand and the complex ion from water to binary solvents.
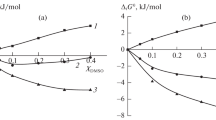
In water—DMSO mixtures, the Δt H 0 values for copper(II) and complex ions were found to change in similar ways, and their contributions to the reaction heat effects compensate each other to a large extent. Thus, the reaction enthalpy change due to solvent composition variation is caused mainly by the changes in ligand solvation enthalpies.
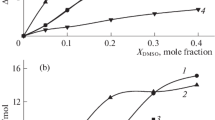
In aqueous ethanol and acetone solutions, the changes in Δt H 0 for all reagents influence the heat effect equally.
Zusammenfassung
In Gemischen von Wasser mit Ethanol, Aceton und Dimethylsulfoxid wurden die Wärmeeffekte der Bildungsreaktionen von Ethylendiamminkupfer(II)-komplexen kalorimetrisch bestimmt. Die Ergebnisse wurden mit Hilfe der Transferenthalpie (Δt H 0) von Komplexbildner, Ligand und Komplexion von Wasser in binäre Lösungsmittel gedeutet.
In einem Wasser-DMSO-Gemisch wurde für die Δt H 0 von Cupfer(II) und den Komplexionen eine ähnliche Änderung festgestellt und ihre Beiträge zu den Reaktionswärmeeffekten kompensieren einander in großem Maße. Somit wird die Änderung der Reaktionsenthalpie bei der Änderung der Lösungsmittelzusammensetzung hauptsächlich durch die Änderung der Solvatationsenthalpien der Liganden verursacht.
In wäßriger Lösung von Ethanol und Aceton wird der Wärmeeffekt durch die Δt H 0-Änderung aller Reagenzien gleich beeinflußt.
Similar content being viewed by others
References
V. A. Shormanov, Complex formation in non-aqueous solutions Moscow, Nauka 1989, p. 256.
G. A. Krestov and V. A. Sharnin, Abstracts of the XXI International Conference on Solution Chemistry. Ottawa 1990, p. 57.
V. A. Sharnin, V. N. Markov, A. V. Nishchenkov, V. A. Shormanov and G. A. Krestov, Abstr. XIV Mendeleyev Congr. Gen. Appl. Chem. Moscow. Nauka 1989, Vol. 1, p. 148.
S. V. Mikheev, V.A. Sharnin, V. A. Shormanov and M. N. Talanova, J. Thermal Anal.
S. V. Mikheev, V. A. Sharnin, A. V. Nishchenkov and V. A. Shormanov, Russian J. Phys. Chem., 66 (1992) 561.
J. Hine and K. W. Narducy, J. Amer. Chem. Soc., 95 (1973) 3362.
V. N. Markov, V. A. Sharnin, V. A. Shormanov and G. A. Krestov, Rev. USSR Acad. Sci, 300 (1988) 1403.
A. V. Nishchenkov and V. A. Sharnin, Abstr. VI Mendeleyev Discussion. Kharkov 1983, p. 91.
A. V. Nishchenkov, V. A. Sharnin, V. A. Shormanov and G. A. Krestov, Russian J. Phys. Chem., 64 (1990) 254.
V. A. Shormanov, V. A. Sharnin and G. A. Krestov, Russian J. Phys. Chem., 53 (1979) 1421.
S. V. Mikheev, S. F. Ledenkov, V. A. Sharnin and V. A. Shormanov, Russian J. Coord. Chem., 19 (1993) 800.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sharnin, V.A. Thermochemistry of formation of copper(II)-ethylenediamine complexes and solvation of reagents in aqueous organic solvents. Journal of Thermal Analysis 45, 721–728 (1995). https://doi.org/10.1007/BF02548887
Issue Date:
DOI: https://doi.org/10.1007/BF02548887