Abstract
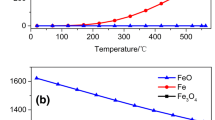
[Fe(N2H4)2(CH3COO)2] was synthesised and characterized for the first time by chemical analysis, magnetic measurements, electronic and IR spectral studies. Its thermal reactivity was ascertained by thermogravimetric (TG) and derivative thermogravimetric (DTG) techniques and it has been concluded that unlike some other metal carboxylate hydrazinates, it does not show any autocatalytic behaviour. The decomposition was also subjected to kinetic analysis using the equations of Coats-Redfern and Horowitz-Metzger by the method of weighted least-squares.
Zusammenfassung
[Fe(N2H4)2(CH3COO)2] wurde erstmalig hergestellt und mittels chemischer Analyse, magnetischen Messungen sowie Elektronen- und IR-Spektraluntersuchungen untersucht. Mit Hilfe von TG- und DTG-Techniken wurde dessen thermische Reaktivität ermittelt und man kam zu der Schlußfolgerung, daß es im Gegensatz zu einigen anderen Metallkarboxy-lathydrazinaten keinerlei autokatalytisches Verhalten zeigt. Unter Anwendung der Gleichungen von Coats-Redfern und Horowitz-Metzger wurde die Zersetzung auch einer kinetischen Analyse unterzogen.
Similar content being viewed by others
References
G.V. Mahesh and K.C. Patil, Thermochim. Acta, 93 (1986) 153.
K.C. Patil, Proc. Indian Acad. Sci. (Chem. Sci.), 96 (1986) 459.
A.I. Vogel, A Text Book of Quantitative Inorganic Analysis, ELBS and Longman, London, 1978.
B.N. Figgis and J. Lewis, Prog. Inorg. Chem., 6 (1964) 185.
A.B. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1968.
L. Sacconi and A. Sabatini, J. Inorg. Nucl. Chem., 25 (1963) 1389.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley, New York, 1978.
K.C. Patil, D. Gajapathy and K. Kishore, Thermochim. Acta, 52 (1982) 113.
K.L. Mampal, Z. Phys. Chem., 187 (1940) 43, 235.
K.K. Aravindakshan and K. Muraleedharan, Reactivity of Solids, 8 (1990) 91.
A.W. Coats and J.P. Redfern, Nature, 201 (1964) 68.
H.H. Horowitz and G. Metzger, Anal. Chem., 35 (1963) 1464.
P.M. Madhusudanan, P.N.K. Nambissan and C.G.R. Nair, Thermochim. Acta, 9 (1979) 149.
A.A. Frost and R.G. Pearson, Kinetics and Mechanism, Wiley, New York, 1961.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jiji, E.R., Aravindakshan, K.K. Synthesis, characterization and thermal analysis of [Fe(N2H4)2(CH3COO)2]. Journal of Thermal Analysis 44, 65–71 (1995). https://doi.org/10.1007/BF02547134
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02547134