Abstract
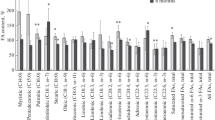
Four cyclopropene fatty acids, having the double bond of the cyclopropene ring at the 8,9, 9,10, 10,11 and 11,12 positions, respectively, were tested as inhibitors of stearic acid desaturation by the desaturase enzyme system of hen liver. The first three were powerful inhibitors, but the last was not. The cyclopropene acids with the 9,10 and 10,11 double bonds were equally strong inhibitors, while the acid with the 8,9 double bond was less effective. To account for the specificity of those cyclopropene fatty acids in which the C9 or C10 carbon atom is included in the cyclopropene ring, it is suggested that the conformation and structure of the CoA derivatives of these acids is such that they can irreversibly occupy the site on the enzyme responsible for 9,10-desaturation.
Similar content being viewed by others
References
Allen, E., A.R. Johnson, A.C. Fogerty, J.A. Pearson and F.S. Shenstone, Lipids 2:419 (1967).
Johnson, A.R., A.C. Fogerty, J.A. Pearson, F.S. Shenstone and A.M. Bersten, Ibid. 4:265 (1969).
Raju, P.K., and R. Reiser, J. Biol. Chem. 242:379 (1967).
Brett, D., D. Howling, L.J. Morris and A.T. James, Arch. Biochem. Biophys. 143:535 (1971).
Pande, S.V., and J.F. Mead, J. Biol. Chem. 245:1856 (1970).
James, A.T., P. Harris and J. Bezard, Eur. J. Biochem. 3:318 (1968).
Fogerty, A.C., A.R. Johnson, J.A. Pearson and F.S. Shenstone, JAOCS 42:885 (1965).
Kircher, H.W., Ibid. 41:4 (1964).
Carroll, K.K., J. Lipid Res. 2:135 (1961).
Mićović, V.M., and M.L. Mihailović, J. Org. Chem. 18:1190 (1953).
Johnson, A.R., K.E. Murray, A.C. Fogerty, B.H. Kennett, J.A. Pearson and F.S. Shenstone, Lipids 2:316 (1967).
Baumann, W.J., and H.K. Mangold, J. Org. Chem. 29:3055 (1964).
Baumann, W.J. and H.K. Mangold, J. Lipid Res. 9:287 (1968).
Gensler, W.J., K.W. Pober, D.M. Solomon and M.B. Floyd, J. Org. Chem. 35:2301 (1970).
Schneider, E.L., S.P. Loke and D.T. Hopkins, JAOCS 45:585 (1968).
Johnson, A.R., J.A. Pearson, F.S. Shenstone and A.C. Fogerty, Nature 214:1244 (1967).
Gensler, W.J., M.B. Floyd, R. Yanase and K.W. Pober, J. Amer. Chem. Soc. 92:2472 (1970).
Williams, J.L. and D.S. Sgoutas, J. Org. Chem. 36:3064 (1971).
Author information
Authors and Affiliations
About this article
Cite this article
Fogerty, A.C., Johnson, A.R. & Pearson, J.A. Ring position in cyclopropene fatty acids and stearic acid desaturation in hen liver. Lipids 7, 335–338 (1972). https://doi.org/10.1007/BF02532651
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02532651