Abstract
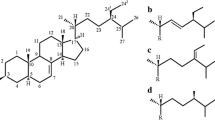
Hydrogenation of stigmasterol yields mixtures of the three title sterols. Stigmasterol was clearly separated from the other two by gas liquid chromatography; a standard curve was constructed relating the height of its peak to its concentration in the mixture. The total unsaturation was measured by a dibromopyridine sulfate-thiosulfate procedure. The unsaturation contributed by previously determined stigmasterol was subtracted from the total to leave that contributed by sitosterol, whose concentration in the mixture was then calculated. The amount of stigmastanol, whose presence was qualitatively determined by thin layer chromatography after bromination of the mixture on the plate, was obtained by difference. When suitable corrections were made, based on analysis of known mixtures, the concentrations of three sterols in hydrogenation mixtures could be calculated.
Similar content being viewed by others
References
Kircher, H.W., and F.U. Rosenstein, Lipids 8:101 (1973).
Patterson, G.W., Anal. Chem. 43:1165 (1971).
Rosanski, A. Ibid. 38:36 (1966).
Grunwald, C., Anal. Biochem. 34:16 (1970).
Ikekawa, N., R. Watanuki, K. Tsuda and K. Sakai, Anal. Chem. 40:1139 (1968).
Hershberg, E.B., E. Oliveto, M. Rubin, H. Staeudle and L. Kuhlen, J. Amer. Chem. Soc. 73:1144 (1951).
Kircher, H.W., and F.U. Rosenstein, Lipids 8:97 (1973).
Ikan, R., S. Harel, J. Kashman and E.D. Bergmann, J. Chromatogr. 14:504 (1964).
Cargill, D.I., The Analyst 87:865 (1962).
Barton, D.H.R., and A.J. Head, J. Chem. Soc. 1956:932.
Khatletskii, A.M., and I.M. Yurist, Żhur. Obsch. Khim. 24:535 (1954); Chem. Abstr. 49:6288 (1955).
Perelman, Y.M., and G.F. Gavrilin, Żhur. Analit. Khim. 18:529 (1963).
Rowe, R.G., C.C. Furnas and H. Bliss, Ind. Eng. Chem., Anal. Ed. 16:371 (1944).
Byrne, R.E., Jr., and J.B. Johnson, Anal. Chem. 28:126 (1956).
Allais, J.P., and M. Barbier, Qual. Plant. Mater. Veg. 16:215 (1968).
Kammereck, R., W.H. Lee, A. Paliokas and G.J. Schroepfer, Jr., J. Lipid Res. 8:282 (1967).
Azarnoff, D.L., and D.R. Tucker, Biochim. Biophys. Acta 70:589 (1963).
Rosenfeld, R.S., Anal. Biochem. 12:483 (1965).
Author information
Authors and Affiliations
Additional information
Contribution No. 1902, Arizona Agricultural Experiment Station.
About this article
Cite this article
Rosenstein, F.U., Kircher, H.W. Analysis of mixtures of stigmasterol, stigmastanol and sitosterol. Lipids 8, 107–110 (1973). https://doi.org/10.1007/BF02531805
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02531805