Abstract
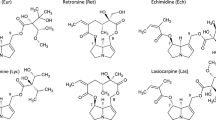
Monocrotaline and trichodesmine are structurally closely related pyrrolizidine alkaloids (PAs) exhibiting different extrahepatic toxicities, trichodesmine being neurotoxic (LD50 57 μmol/kg) and monocrotaline pneumotoxic (LD50 335 μmol/kg). We have compared certain physicochemical properties and metabolic activities of these two PAs in order to understand the quantitative and qualitative differences in toxicity. Both PAs were metabolized in the isolated, perfused rat liver to highly reactive pyrrolic dehydroalkaloids that appear to be responsible for the toxicity of PAs. More dehydrotrichodesmine (468 nmol/g liver) than dehydromonocrotaline (116 nmol/g liver) was released from liver into perfusate on perfusion for 1 hr with 0.5 mM of the parent PA. Dehydrotrichodesmine had a significantly longer aqueous half-life (5.4 sec) than that of dehydromonocrotaline (3.4 sec). In vivo, significantly higher levels of bound pyrroles were found in the brain 18 hr after injection of trichodesmine (25 mg/kg; i.p) than were seen following either an equal dose (25 mg/kg; i.p.) or an equitoxic dose (90 mg/kg; i.p.) of monocrotaline. Trichodesmine had a higher partition coefficient than monocrotaline for both chloroform and heptane, indicating its greater lipophilicity. The pKa of trichodesmine (7.07) was only slightly higher than that of monocrotaline (pKa 6.83), suggesting that a difference in degree of ionization was not a major factor affecting the relative ability of the dehydroalkaloids to cross the blood-brain barrier. We conclude that the greater lethality and neurotoxicity of trichodesmine compared to monocrotaline is due to two structural characteristics: (i) steric hindrance at position 14 of dehydrotrichodesmine results in greater resistance to hydrolysis, allowing more to be released from the liver and to be delivered to the brain; (ii) the larger isopropyl substituent at position 14 of dehydrotrichodesmine renders the molecule more lipophilic, leading to greater penetration of the brain.
Similar content being viewed by others
References
Mattocks, A.R. (1986). Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, London.
Smith, L.W., and Culvenor, C.C.J. 1981. Plant sources of hepatotoxic pyrrolizidine alkaloids, J. Nat. Prod. 44:129–152.
Robins D.J. (1982). The pyrrolizidine alkaloids, Fortschr. Chem. Org. Naturst, 41:115–203.
Huxtable R.J. (1989). Human health implications of pyrrolizidine alkaloids and herbs containing them. Pages 41–86,in Cheeke, P.R. (ed.), Toxicants of Plant Origin, Vol I: Alkaloids, CRC Press, Boca Raton, Florida.
Huxtable, R.J. (1993). Hepatic nonaltruism and pulmonary toxicity of pyrrolizidine alkaloids. Pages 215–239,in Gram, T.E. (ed.), Metabolic Activation and Toxicity of Chemical Agents to Lung Tissue and Cells, Pergamon Press, New York.
Mattocks A.R. 1972. Acute hepatotoxicity and pyrrolic metabolites in rats dosed with pyrrolizidine alkaloids. Chem.-Biol. Interact. 5:227–242.
Huxtable R.J., and Wild S.L. 1994. Relationship between in vitro metabolism of pyrrolizidine alkaloids and extrahepatic toxicity in vivo, Proc. Western Pharmacol. Soc. 37:109–111.
Williams D.E., Reed R.L., Kedzierski B., Dannan G.A., Guengerich F.P., and Buhler D.R. (1989). Bioactivation and detoxication of the pyrrolizidine alkaloid senecionine by cytochrome P-450 enzymes in rat liver, Drug Metab. Disp. 17:387–392.
Miranda C.L., Reed R.L., Guengerich F.P., and Buhler D.R. 1991. Role of cytochrome P450IIIA4 in the metabolism of the pyrrolizidine alkaloid senecionine in human liver, Carcinogenesis, 12:515–519.
Mattocks A.R., and Jukes R. 1990. Trapping and measurement of short-lived alkylating agents in a recirculating flow system, Chem.-Biol. Interact. 76:19–30.
Candrian U., Luthy J., and Schlatter C. 1985. In vivo covalent binding of retronecine-labelled [3H]seneciphylline and [3H]senecionine to DNA of rat liver, lung and kidney, Chem.-Biol. Interact. 54:57–69.
Huxtable R.J., Bowers R., Mattocks A.R., and Michnicka M. 1991. Sulfur conjugates as putative pneumotoxic metabolites of the pyrrolizidine alkaloid, monocrotaline. Pages 605–612,in Witmer, C.M., Snyder, R.R., Jollow, D.J., Kalf, G.F., Kocsis, J.J., and Sipes, I.G. (eds.), Biological Reactive Intermediates Vol. IV: Molecular and Cellular Effects and their Impact on Human Health, Plenum Press, New York.
Culvenor, C.C.J., Dann A.T., and Dick A.T. 1962. Alkylation as the mechanism by which the hepatotoxic pyrrolizidine alkaloids act on cell nuclei, Nature (London), 195:570–573.
Mattocks A.R., Croswell S., Jukes R., and Huxtable R.J. 1991. Identity of a biliary metabolite formed from monocrotaline in isolated, perfused rat liver, Toxicon. 29:409–415.
Adams R., and Rogers E.F. 1939. The structure of monocrotaline, the alkaloid in Crotalaria spectabilis and Crotalaria retusa, J. Amer. Chem. Soc. 61:2815–2819.
Mattocks A.R., Jukes R., and Brown J. 1989. Simple procedures for preparing putative toxic metabolites of pyrrolizidine alkaloids, Toxicon, 27:561–567.
Yan C.C., and Huxtable R.J. 1994. Quantitation of the hepatic release of metabolites of the pyrrolizidine alkaloid, monocrotaline, Toxicol. Appl. Pharmacol. 127:58–63.
Mattocks A.R., and White I.N.H. 1970. Estimation of metabolites of pyrrolizidine alkaloids in animal tissues, Anal. Biochem. 38:529–535.
Yan C.C., and Huxtable R.J. 1995. Relationship between glutathione concentration and metabolism of the pyrrolizidine alkaloid, monocrotaline, in the isolated, perfused liver, Toxicol. Appl. Pharmacol. 130:132–139.
Robertson K.A. 1982. Alkylation of N2 in deoxyguanosine by dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine alkaloid monocrotaline, Cancer Res. 42:8–14.
Niwa H., Ogawa T., Okamoto O., and Yamada K. 1991. Alkylation of nucleosides by dehydromonocrotaline, the putative toxic metabolite of the carcinogenic pyrrolizidine alkaloid monocrotaline, Tetrahedron Letters, 32:927–930.
Petry T., Bowden G.T., Huxtable R.J. and Sipes I.G. 1984. Characterization of hepatic DNA damage induced by the pyrrolizidine alkaloid monocrotaline, Cancer Res. 44:1505–1509.
Kay, J.M., and D. Heath 1969. Crotalaria Spectabilis: The Pulmonary Hypertension Plant, Thomas, Springfield, IL.
Huxtable R.J. 1990. Activation and pulmonary toxicity of pyrrolizidine alkaloids, Pharmacology and Therapeutics, 47:371–389.
Hayashi, Y. and J.J. Lalich 1967. Renal and pulmonary alterations induced in rats by a single injection of monocrotaline, Proc. Soc. Exp. Biol. Med. 124:392–396. (Abstract)
Carrillo L., and Aviado D. 1969. Monocrotaline-induced pulmonary hypertension and p-chlorophenylalanine (PCPA), Laboratory Investigation, 20:243–248.
Blaustein M.P. 1988. Calcium transport and buffering in neurons, Trends Neurol. Sci. 11:438–443.
Ismailov N.I., Madzhidov N.M., Magrupov A.L., Makhkamov G.M., and Mukminova S. 1970. Clinical signs, diagnosis and treatment of Trichodesma toxicosis (alimentary toxic encephalopathy), Meditsina (Tashkent, Uzbek SSR), 85
IARC 1975. Pyrrolizidine alkaloids, IARC Monograph, 10:265–343.
Glowaz S.L., Michnika M. and Huxtable R.J. 1992. Detection of a reactive pyrrole in the hepatic metabolism of the pyrrolizidine alkaloid, monocrotaline, Toxicol. Appl. Pharmacol. 115:168–173.
Mattocks A.R. 1968. Toxicity of pyrrolizidine alkaloids, Nature (London), 217:723–728.
Yan C.C. and Huxtable R.J. 1995. The relationship between the concentration of the pyrrolizidine alkaloid monocrotaline and the pattern of metabolites released from the isolated liver, Toxicol. Appl. Pharmacol. 130:1–8.
Cooper R.A. and Huxtable R.J. 1996. A simple procedure for determining the aqueous half-lives of pyrrolic metabolites of pyrrolizidine alkaloids Toxicon, in press.
Author information
Authors and Affiliations
Additional information
Special issue dedicated to Dr. Kinya Kuriyama
Rights and permissions
About this article
Cite this article
Huxtable, R.J., Yan, C.C., Wild, S. et al. Physicochemical and metabolic basis for the differing neurotoxicity of the pyrrolizidine alkaloids, trichodesmine and monocrotaline. Neurochem Res 21, 141–146 (1996). https://doi.org/10.1007/BF02529131
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02529131