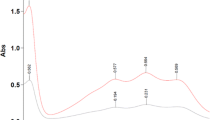
Abstract
The capability of a compound or of a mixture of compounds to quench peroxyl radicals was measured by analyzing the kinetics of the competition of a parallel reaction where peroxyl radicals bleach the carotenoid crocin. This kinetic approach, originally described for the analysis of antioxidants reacting with hydroxyl radicals in water, was modified by both decreasing the polarity of the solvent, thus allowing the analysis of lipophilic compounds, and by substituting a source of peroxyl radicals for the hydroxyl radical generating system. Single compounds as well as complex mixtures were analyzed by kinetic data processing. Overall antioxidant capacity, relative to that of α-tocopherol or of its soluble analog Trolox C, was calculated. As examples of the use of this test, the antioxidant capacities of a crude rosemary extract, Maillard reaction products, and virgin olive oils were measured.
Similar content being viewed by others
References
Porter, N.A., Autoxidation of Polyunsaturated Fatty Acids: Initiation, Propagation, and Product Distribution (basic chemistry), inMembrane Lipid Oxidation, Vol. I, edited by C. Vigo-Pelfrey, CRC Press, Boca Raton, 1990, pp. 33–62.
Chan, H.W.S., The Mechanism of Autoxidation, inAutoxidation of Unsaturated Lipids, Academic Press, London, 1987.
Simic, M., and M. Karel,Autoxidation in Food and Biological Systems Plenum Press, New York, 1980.
Terao, J., Reactions of Lipid Hydroperoxides, inMembrane Lipid Oxidation, Vol. I, edited by C. Vigo-Pelfrey, CRC Press, Boca Raton, 1990, pp. 219–238.
Frankel, E.N., In Search for Better Methods to Evaluate Natural Antioxidants and Oxidative Stability in Food Lipids,Trends in Food Science and Technology 4: 220–225 (1993).
Rossel, J.B., Measurement of Rancidity, inRancidity in Foods, edited by J.C. Allen and R.J. Hamilton, Elsevier, Amsterdam, 1983, pp. 21–45.
Ragnarsson, J.O., and T.P. Labuza, Accelerated Shelf-Life Testing for Oxidative Rancidity in Foods—A Review.Food Chem. 2: 291–298 (1977).
Halliwell, B., and M.C. Gutteridge, Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview.Meth. in Enzmol. 186: 1–85 (1990).
Addis, P.B., and G.J. Warner, The Potential Health Aspects of Lipid Oxidation Products in Food, inFree Radicals and Food Additives, edited by O.I. Aruoma and B. Halliwell, Taylor and Francis, London, 1991, pp. 77–119.
Ames, B.N., M.K. Shigenaga, and T.M. Hagen, Oxidants, Antioxidants, and the Degenerative Diseases of Aging,Proc. Natl. Acad. Sci. 90: 7915–7922 (1993).
Diplock, A.T. Antioxidants Nutrients and Disease Prevention: An Overview,Am. J. Clin. Nutr. 53: 189S-193S (1991).
Block, G., The Data Support a Role for Antioxidants in Reducing Cancer Risk.Nutr. Rev. 50: 207–213 (1992).
Gey, K.F., P. Puska, P. Jordan, and U.K. Moser, Inverse Correlation Between Plasma Vitamin E and Mortality from Ischemic Heart Disease in Cross-Cultural Epidemiology,Am. J. Clin. Nutr. 53: 326–334S (1991).
Fedeli, E.: Lipids of Olives,Prog. Chem. Fats Other Lipids 15: 57–74 (1977).
Papadopoulos, G., and D. Boskou, Antioxidant Effect of Natural Phenols on Olive Oil,J. Am. Oil Chem. Soc., 68: 669–671 (1991).
Läubli, M.W., and P.A. Bruttel, Determination of the Oxidative Stability of Fats and Oils: Comparison Between the Active Oxygen Method (AOAC cd 12–57) and the Rancimat Method,63: 792–795 (1986).
Hasegawa, K., and L.K. Patterson, Pulse Radiolysis in Model Systems: Formation and Behaviour of Peroxy Radicals in Fatty Acids,Phtochem. Photobiol. 28: 817–823 (1978).
Pryor, W.A., The Role of Free Radical Reactions in Biological Systems, inFree Radicals in Biology, Vol. I, edited by W.A. Pryor, Academic Press, New York, 1976, pp. 1–49.
Ursini, F., M. Maiorino, and A. Sevanian, Membrane Hydroperoxides, inOxidative Stress: Oxidants and Antioxidants, edited by H. Sies, Academic Press, London, 1991, pp. 319–336.
Barclay, L.R.C., K.A. Baskin, S.J. Locke, and M.R. Vinquist, Absolute Rate Constants for Lipid Peroxidation and Inhibition in Model Biomembranes.Can. J. Chem. 67: 1366–1369 (1989).
Nagaoka, S., Y. Okauchi, S. Urano, U. Nagashima, and K. Mukai, Kinatic andab initio Study of the Prooxidant Effect of Vitamin E. Hydrogen Abstraction from Fatty Acid Esters and Egg Yolk Lecithin,J. Am. Chem. Soc. 112: 8921–8924 (1990).
Small, R.D. Jr., J.C. Scaino, and L.K. Patterson, Radical Processes in Lipids. A Laser Photolysis Study oft-Butoxy Radical Reactivity Toward Fatty Acids,Photochem. Photobiol. 29: 49–51 (1979).
Gardner, H.W., D. Weisleder, and R. Kleiman, Formation oftrans-12,13-Epoxy-9-Hydroperoxy-trans-10-Octadecenoic Acid from 13-l-Hydroperoxy-cis-9,trans-11-Octadecadienoic Acid Catalyzed by Either a Soybean Extract or Cysteine-FeCl3,Lipids 13: 246–255 (1978).
Labeque, R., and L.J. Marnett, Reaction of Hematin with Allylic Fatty Acid Hydroperoxides: Identification of Products and Implication for Pathways of Hydroperoxide-Dependent Epoxydation of 7,8-Dihydroxy-7,8 dihydrobenzo [a] pyrene.Biochemistry, 27: 7060–7070 (1988).
Lillard, D.A., and E. Day. Degradation of Monocarbonyls from Autoxidizing Lipids,J. Am. Oil Chem. Soc., 41: 549–554 (1964).
Esterbauer, H., H. Zollner, and R. Jörg Schaur, Aldehydes Formed by Lipid Peroxidation: Mechanisms of Formation, Occurrence, and Determination, inMembrane Lipid Oxidation, Vol. I, edited by C. Vigo-Pelfrey, CRC Press, Boca Raton, 1990, pp. 239–268.
Mukai, K., K. Sawada, Y. Kohno, and J. Terao, Kinetic Study of the Prooxidant Effect of Tocopherol. Hydrogen Abstraction from Lipid Hydroperoxides by Tocopheroxyls in Solution,Lipids 28: 747–752, (1993).
Maiorino, M., A. Roveri, and F. Ursini, Antioxidant Effect of Ebselen (PZ51): Peroxidase Mimetic Activity on Phospholipid and Cholesterol Hydroperoxides vs. Free Radical Scavenger Activity,Arch. Biochem. Biophys. 295: 404–409 (1992).
Bors, W., C. Michel, and M. Saran, Inhibition of Bleaching of the Carotenoid Crocin, A Rapid Test for Quantifying Antioxidant Activity,Bioch. Biophys. Acta 796: 312–319 (1984).
Bors, W., M. Erben-Russ, and M. Saran, Fatty Acid Peroxyl Radicals: Their Generation and Reactivities,Biolectrochem. Bioenergetics 18: 37–49 (1987).
Pryor, W.A., J.A. Cornicelli, L.J. Devall, B. Tait, B.K. Trivedi, D.T. Witiak, and M. Wu, A Rapid Screening Test to Determine the Antioxidant Potencies of Natural and Synthetic Antioxidants,J. Org. Chem. 58: 3521–3532 (1993).
von Sonntag, C., and H.P. Schuchmann, Pulse Radiolysis,Meth. in Enzymol. 233: 3–20 (1994).
Franzke, C., and H. Iwainsky, Zur Antioxydativen Wirksamkeit der Melanoidine,Deut. Lebensm.- Rundschau. 50: 251–254 (1954).
van Amsterdam, F.T.M., A. Roveri, M. Maiorino, E. Ratti, and F. Ursini, Lacidipine: A Dihydropyridine Calcium Antagonist with Antioxidant Activity,Free Radical Biol. Med. 12: 183–187 (1992).
Ursini, F., M. Maiorino, P. Morazzoni, A. Roveri, and G. Pifferi, A Novel Antioxidant Flavonoid (IdB 1031) Affecting Molecular Mechanisms of Cellular Activation,Free Rad. Biol. Med. 16: 547–553 (1994).
Eichner, K., Antioxidative Effect of Maillard Reaction Intermediates,Prog. Fd. Nutr. Sci. 5: 441–451 (1981).
Lingnert, H., and C.E. Eriksson, Antioxidative Effect of Maillard Reaction Products,Prog. Fd. Nutr. Sci. 5: 453–466 (1981).
Nergiz, C., and K. Unal, Determination of Phenolic Acids in Virgin Olive Oil,Food Chem. 39: 237–245 (1991).
Montedoro, G., M. Servili, M. Baldioli, and E. Miniati, Simple and Hydrolyzable Phenolic Compouns in Virgin Olive Oil 1—Their Extraction, Separation, and Quantitative and Semiquantitative Evaluation by HPLC,J. Agric. Food. Chem. 40: 1571–1578 (1992).
Author information
Authors and Affiliations
About this article
Cite this article
Tubaro, F., Micossi, E. & Ursini, F. The antioxidant capacity of complex mixtures by kinetic analysis of crocin bleaching inhibition. J Am Oil Chem Soc 73, 173–179 (1996). https://doi.org/10.1007/BF02523891
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02523891