Abstract
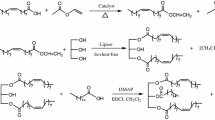
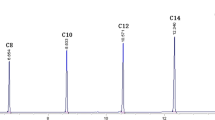
Lipases that display high regioselectivities and broad substrate tolerance were used as catalysts for the efficient esterification of glycerol under the conditions of irreversible acyl transfer. A variety of unsaturated fatty acids, such as oleic, linoleic, erucic, ricinolic, hydroxystearic and coriolic acid, were used for this purpose in the form of their vinyl esters. Suitable biocatalysts were chosen on the basis of systematic screening experiments regarding their regioselectivities (RE) and substrate tolerances. Distinct differences were found and expressed in numerical RE values as a measure for differences of these biocatalysts as being specific, selective, and nonspecific. Based on these experiments, a variety of molecules were synthesized on a preparative scale (>150 mmol) in good yield (ca.85%) and with high regioisomerical purities (>95% RE).
Similar content being viewed by others
References
Van Deenen, L.L.M., and G.H. De Haas, The Substrate Specifity of Phospholipase A,Biochim. Biophys. Acta 70:538–553 (1963).
Ahmed, F.U., Efficient Synthesis of Fatty Monoglyceride Sulfates from Fatty Acids and Fatty Acid Methyl Esters,J. Am. Oil Chem. Soc. 67:8–14 (1990).
Kumar, R., and J.D. Billimoria, Gastric Ulceration and the Concentration of Salicylate in Plasma in Rats After Administration of14C-labeled Aspirin and Its Synthetic Triglyceride, 1,3-dipalmitoyl-2(2′-acetoxy-[14C]carboxyben-zoyl)-glycerol,J. Pharm. Pharmacol. 30:754–758 (1978).
Paris, G.Y., D.G. Cimon, D.L. Garmaise, L. Swett, J.W. Carter, and P.Y. Young, Glycerides as Prodrugs. 3. Synthesis and Antiinflammatory Activity of [1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetyllglycerides (indomethacin glycerides),J. Med. Chem. 23:9–13 (1980).
Jacob, J.N., G.W. Hesse, and V.E. Shashoua, γ-Aminobutyric Acid Esters. 3. Synthesis, Brain Uptake, and Pharmacological Properties of C-18 Glyceryl Lipid Esters of GABA with Varying Degree of Unsaturation,J. Med. Chem. 30:1573–1576 (1987).
Jacob, J.N., G.W. Hesse, and V.E. Shashoua, Synthesis, Brain Uptake, and Pharmacological Properties of a Glyceryl Lipid containing GABA and the GABA-T Inhibitor γ-vinyl-GABA,33:733–736 (1990).
Garzon-Aburbeh, A., J.H. Poupaert, M. Claesen, P. Dumont, and G. Atassi, 1,3-Dipalmitoylglycerol Ester of Chlorambucil as a Lymphotrophic, Orally Administrable Antineoplastic Agent,26:1200–1203 (1983).
Saraiva Goncalves, J.C., J.H. Poupaert, and P. Dumont, Disconnection in Drug Design: Diacylglycerols as Lymphotropic/Celphalotropic Micromolecular Reactors of Drugs. Application tol-Dopa and Chlorambucil,Actual. Chim. Ther. 15:265–267 (1988).
Mantelli, S., P. Speiser, and H. Hauser, Phase Behavior of Diglyceride Prodrugs: Spontaneous Formation of Unilamellar Vesicles,Chem. Phys. Lipids 37:329–343 (1985).
McNeill, G.P., and T. Yamane, Further Improvements in the Yield of Monoglycerides During Enzymatic Glycerolysis of Fats and Oils,J. Am. Oil Chem. Soc. 68:6–10 (1991).
Kido, H., N. Fukussen, K. Ishidoh, and N. Katunuma, Diacylglycerol Amplifies the Inductionin vivo of Tyrosine Aminotransferase and Ornithine Decarboxylase by Glucocorticoid,Biochem. Biophys. Res. Commun. 138:275–282 (1986).
Siegel, D.P., J. Banschbach, D. Alford, H. Ellens, L.J. Lis, P.J. Quinn, P.L. Yeagle, and J. Bentz, Physiological Levels of Diacyglycerols in Phospholipid Membranes Induce Membrane Fusion and Stabilize Inverted Phases,Biochemistry 28:3703–3709 (1989).
Swern, D., inBailey’s Industrial Oil and Fat Products, Vol. 1, Wiley Interscience, New York, 1979, p. 3.
Pratt, C.D., and W.W. Hays, Food Emulsifiers Bring New Highs in Uniformity. I. Four Types Evaluated,Food Eng. 24:109–112 (1952).
Ikeda, I., X.P. Gu, I. Mijamoto, and M. Okahara, Preparation of 1,3-Diacylglycerols and 1-Alkyl-3-Acylglycerols in the Presence of Quaternary Ammonium Salt,J. Am. Oil Chem. Soc. 66:822–824 (1989).
Bentley, P.H., and W. McCrea, An Efficient Synthesis of Symmetrical 1,3-Diglycerides,J. Org. Chem. 35:2082–2083 (1970).
Mattson, F.H., and R.A. Volpenheim, Synthesis and Properties of Glycerides,J. Lipid Res. 3:281–296 (1962).
Howe, R.J., and T. Malkin, An X-Ray and Thermal Examination of the Glycerides. Part XI. The 1:2-Diglycerides, and Further Observations on 1:3-Diglycerides,J. Chem. Soc. 2663–2667 (1951).
Osada, K., K. Takahashi, and M. Hatano, Polyunsaturated Fatty Glyceride Syntheses by Microbial Lipases,J. Am. Oil Chem. Soc., 67:921–922 (1990).
Zaks, A., Enzymatic Production of Monoglycerides,66:484 (1989).
Holmberg, K., and E. Osterberg, Enzymatic Preparation of Monoglycerides in Microemulsion,65:1544–1548 (1988).
Berger, M., K. Laumen, and M.P. Schneider, Enzymatic Esterification of Glycerol I. Lipase-Catalyzed Synthesis of Regioisomerically Pure 1,3-sn-Diacylglycerols,,69:955–960 (1992).
Berger, M., and M.P. Schneider, Enzymatic Esterification of Glycerol II. Lipase-Catalyzed Synthesis of Regioisomerically Pure 1(3)-rac-Monoacylglycerols,69:961–965 (1992).
Berger, M., and M.P. Schneider, Regioselectivity of Lipases in Organic Solvents,Biotechnol. Lett., 13:333–338 (1991).
Lobell, M., and M.P. Schneider, Synthesis of Hydroxycarboxylic Acid Vinyl Esters,Synthesis 375–377 (1994).
Author information
Authors and Affiliations
About this article
Cite this article
Waldinger, C., Schneider, M. Enzymatic esterification of glycerol III. Lipase-catalyzed synthesis of regioisomerically pure 1,3-sn-diacylglycerols and 1 (3)-rac-monoacylglycerols derived from unsaturated fatty acids. J Am Oil Chem Soc 73, 1513–1519 (1996). https://doi.org/10.1007/BF02523518
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02523518