Abstract
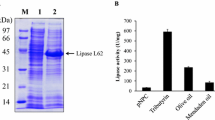
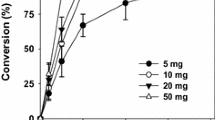
Soapstock (SS) is a by-product of the extraction of oilseeds to produce edible oils. Annual U.S. production exceeds one-half million tons. A representative sample of SS consists of 45.1% water, 10.0% free fatty acids, 10.1% triglycerides, 1.8% diglycerides, 3.6% phosphatidylethanolamine, 2.2% phosphatidylinositol, 2.7% phosphatidylcholine, 14.0% solvent-insolubles and 10.5% other material, which was not characterized. A process has been developed that sequentially employs a nonenzymatic and an enzymatic step to convert the lipid-linked and the free fatty acids of SS to the esters of monohydric alcohols. The first step of the process employed alcohol and potassium hydroxide to transesterify the glyceride-and phosphoglyceride-linked fatty acids of the substrate. Because water inhibited the reaction, it was necessary that the SS be dried before use. Nonetheless, even with some batches of SS with water contents below 1% (weight basis), ester hydrolysis accompanied esterification. Each of five examined simple primary alcohols participated effectively in the transesterification reaction, which proceeded rapidly at room temperature and was essentially complete within 1 h. The average ratio of transesterification to hydrolysis in four examined small primary alcohols was 4:1. However, in methanol this value was 99:1 due to the virtual absence of hydrolysis. Significant transesterification by a secondary alcohol (isopropanol) did not occur at room temperature. The minimum effective molar ratio of alcohol to lipid-linked fatty acids was 20:1. The minimum effective concentration of KOH was between 0.10 and 0.15N. The efficiency of the transesterification reaction exceeded 90% of theoretical maximum. The second step of the process involves lipase-mediated esterification of the free fatty acids in the preparation that are not esterified by the alkaline transesterfication. Of four lipase preparations examined (Novo Lipozyme IM 20 and SP435, and Amano PS30 and CE), only SP-435 catalyzed significant esterification of the free fatty acids. The reaction was not catalyzed by heat-denatured enzyme. In the pH range between 6 and 13.5, the enzyme reaction proceeded best at pH 6, although also well at pH 7. The optimal water concentration was 0.70% (vol/vol). At an enzyme dosage of 1.1% (weight basis, relative to the dry weight of SS present) under optimal conditions and at 42°C, 63% of the free fatty acids in a post-alcoholysis mixture were enzymatically esterified. The addition of molecular sieves did not increase esterification, which was probably retarded by the high viscosities of the reactions. Under the optimal conditions identified here, the degree of conversion of the fatty acids in SS to simple alkyl esters by the combined reaction scheme was 81%. Opportunities exist for further optimization of these reactions.
Similar content being viewed by others
References
Snyder, H.E., and T.W. Kwon,Soybean Utilization, Van Nostrand Reinhold Co., New York, 1987, pp. 110–112.
Anonymous,Soya Bluebook Plus, Soyatech, Inc., Bar Harbor, ME, 1995, p. 262.
Schuch, R., and K.D. Mukherjee, Interesterification of Lipids Using an Immobilizedsn-1,3-Specific Triacylglycerol Lipase,J. Agric. Food Chem. 35:1005–1008 (1987).
Kanasawud, P., S. Phutrakul, S. Bloomer, P. Adlercreutz, and B. Mattiasson, Triglyceride Interesterification by Lipases. 3. Alcoholysis of Pure Triglycerides,Enz. Microb. Technol. 12:959–965 (1992).
Mittelbach, M., Lipase-Catalyzed Alcoholysis of Sunflower Oil,J. Am. Oil Chem. Soc. 67:168–170 (1990).
Linko, Y.-Y., M. Lamsa, A. Huhtala, and O. Rantanen, Lipase Biocatalysis in the Production of Esters,72:1293–1299 (1995).
Goshray, S., and D.K. Bhattacharya, Enzymatic Preparation of Ricinoleic Acid Esters of Long-Chain Monohydric Alcohols and Properties of the Esters,69:85–88 (1992).
Sonntag, N.O.V., Fat Splitting, Esterification, and Interesterification, inBailey’s Industrial Oil and Fat Products, edited by D. Swern, Vol. 2, 4th edn., J. Wiley and Sons, New York, 1982, pp. 97–173.
Formo, M.W., Ester Reaction of Fatty Materials,J. Am. Oil Chem. Soc. 31:548–559 (1954).
Eisenhard, W.C., inFatty Acids in Industry, edited by R.W. Johnson and E. Fritz, Marcel Dekker, Inc., New York, 1989, pp. 139–152.
Isigigur, A., F. Karaosmanoglu, and H.A. Aksoy, Methyl Ester from Safflower Seed Oil of Turkish Origin as a Biofuel for Diesel Engines,App. Biochem. Biotech. 45/46:103–112 (1994).
Freedman, B., E.H. Pryde, and T.L. Mounts, Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils,J. Am. Oil Chem. Soc. 61:1638–1643 (1984).
Bradshaw, G.B., and W.C. Meuly, Preparation of Detergents, U.S. Patent 2,360,822 (1944).
Bloomer, S., P. Adlercreutz, and B. Mattiasson, Facile Synthesis of Fatty Acid Esters in High Yields,Enzyme Microb. Technol. 14:546–552 (1992).
Miller, C., H. Austin, L. Posorske, and J. Gonzlez, Characteristics of an Immobilized Lipase for the Commercial Synthesis of Esters,J. Am. Oil Chem. Soc. 65:927–931 (1988).
Ghoshray, S., and D.K. Bhattacharya, Utilization of Acid Oils in Making Valuable Fatty Products by Microbial Lipase Technology,72:1541–1544 (1995).
Vazquez Lima, R., D.L. Pyle, and J.A. Asenjo, Factors Affecting the Esterification of Lauric Acid Using an Immobilized Biocatalyst: Enzyme Characterization and Studies in a Well-Mixed Reactor,Biotechnol. Bioeng. 46:69–79 (1995).
Norris, R.A., Refining and Bleaching, inBailey’s Industrial Oil and Fat Products, edited by D. Swern, Vol. 2, 4th edn., J. Wiley and Sons, New York, 1982, pp. 253–314.
Stern, R., G. Hillion, P. Gateau, and J.C. Guibet, Preparation of Methyl and Ethyl Esters from Crude Vegetable Oils and Soapstock, inProceedings: World Conference on Emerging Technologies in the Fats and Oils Industry, edited by A.R. Baldwin, American Oil Chemists’ Society, Champaign, 1986, pp. 420–422.
Haas, M.J., D.J. Cichowicz, W. Jun, and K. Scott, The Enzymatic Hydrolysis of Triglyceride-Phospholipid Mixtures in an Organic Solvent,J. Am. Oil Chem. Soc. 72:519–525 (1995).
Haas, M.J., D. Esposito, and D.J. Cichowicz, A Software Package to Streamline the Titrimetric Determination of Lipase Activity,72:1405–1406 (1995).
Wright, H.J., J.B. Segur, H.V. Clark, S.K. Coburn, E.E. Langdon, and R.N. DuPuis, A Report on Ester Interchange,Oil & Soap 21:145–148 (1944).
Feuge, R.O., and A.T. Gros, Modification of Vegetable Oils. VII. Alkali-Catalyzed Interesterification of Peanut Oil with Ethanol,J. Am. Oil Chem. Soc. 26:97–102 (1949).
Sprules, F.J., and D. Price, Production of Fatty Esters, U.S. Patent 2, 494,366 (1950).
Clark, S.J., L. Wagner, M.D. Schrock, and P.G. Piennaar, Methyl and Ethyl Soybean Esters as Renewable Fuels for Diesel Engines,J. Am. Oil Chem. Soc. 61:1632–1638 (1984).
Zaks, A., and A.M. Klibanov, The Effect of Water on Enzyme Action in Organic Media,J. Biol. Chem. 253:8017–8021 (1988).
Hirata, H., K. Higuchi, and T. Yamashina, Lipase-Catalyzed Transesterification in Organic Solvent: Effects of Water and Solvent, Thermal Stability and Some Applications,J. Biotechnol. 14:157–167 (1990).
Adlercreutz, P., On the Importance of the Support Material for Enzymatic Reactions in Organic Media,Eur. J. Biochem. 199:609–614 (1991).
Valivety, R.H., P.J. Halling, A.D. Peilow, and A.R. Macrae, Lipases from Different Sources Vary Widely in Dependence of Catalytic Activity on Water Activity,Biochem. Biophys. Acta 1122:143–146 (1992).
Valivety, R.H., P.J. Halling, and A.R. Macrae, Water as a Competitive Inhibitor of Lipase-Catalysed Esterification in Organic Media,Biotechnol. Lett. 15:1133–1138 (1993).
Svensson, I., E. Wehtje P. Adlercreutz, and B. Mattiasson, Effects of Water Activity on Reaction Rates and Equilibrium Positions in Enzymatic EsterificationsBiotechnol. Bioeng. 44:549–556 (1994).
Haas, M.J., K. Scott, W. Jun, and G. Janssen, Enzymatic Phosphatidylcholine Hydrolysis in Organic Solvents: An Examination of Selected Commercially Available Lipases,J. Am. Oil Chem. Soc. 71:483–490 (1994).
Author information
Authors and Affiliations
About this article
Cite this article
Haas, M.J., Scott, K.M. Combined nonenzymatic-enzymatic method for the synthesis of simple alkyl fatty acid esters from soapstock. J Am Oil Chem Soc 73, 1393–1401 (1996). https://doi.org/10.1007/BF02523502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02523502