Abstract
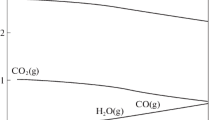
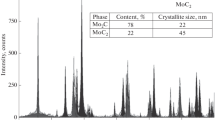
It was shown that the carbides of transition metals are effective catalysts of oxidation reactions on account of their metal-like nature, combined with their high chemical and thermal stability. The results from systematic investigations into the catalytic characteristics of the carbides in the oxidation of hydrogen, carbon monoxide, and ammonia and in the oxidative coupling of methane (OCM) are examined. The first two reactions are total oxidation processes, and the oxidation of ammonia is a selective oxidation process. The oxidative coupling of methane is a heterogeneous-homogeneous process and represents a prospective method for the direct transformation of methane into higher hydrocarbons.
Similar content being viewed by others
References
E. K. Storms,The Refractory Carbides, Academic Press, New York (1967).
L. E. Toth,Transition Metal Carbides and Nitrides, Academic Press, New York (1971).
L. F. Shakelford,Introduction to Materials Science for Engineers, Macmillan, New York (1988).
N. I. Il'chenko,Kinet. Katal.,14, No. 4, 976–980 (1973).
N. I. Il'chenko,Kinet. Katal.,18, No. 1, 153–163 (1977).
S. T. Oyama and G. L. Haller,Catalysis: Spec. Period. Rep.,5, 333–365 (1981).
S. T. Oyama,Catal Today,15, 179–200 (1992).
L. Leclercq,Surface Properties and Catalysis by Nonmetals, Reidel, Dordrecht (1983).
S. J. Karf, J. A. Ross, N. A. de Bruijn, et al.,Catal. Today,2, 535 (1988).
J. S. Lee and S. T. Oyama,Catal. Rev. Sci. Eng.,30, 249 (1988).
L. R. Anderson,Appl. Catal.,47, 177–196 (1989).
M. Baerns, van der Wiele, and J. R. H. Anderson,Catal. Today,4, 471 (1989).
J. H. Lunsford,Catal. Today,6, 235 (1990).
K. Aika, N. Fujimoto, M. Kobayashi, and E. Iwamatsu,J. Catal.,127, 1–8 (1991).
N. I. Il'chenko, E. M. Malyshev, Yu. D. Pankrat'ev, and G. I. Golodets,Kinet. Katal.,19, No. 3, 639–644 (1978).
N. P. Chebotarev, N. I. Il'chenko, and G. I. Golodets,Teor. Éksp. Khim.,12, No. 3, 361–366 (1976).
D. Brennan, D. O. Hayward, and B. W. M. Trapnell,Proc. Roy. Soc. London, A,256, 81–105 (1960).
G. K. Boreskov,Heterogeneous Catalysis [in Russian], Nauka, Moscow (1986).
N. I. Il'chenko,Usp. Khim.,41, No. 1, 84–95 (1972).
N. I. Il'chenko,Kinet. Katal.,14, No. 3, 798–801 (1973).
L. G. Svintsova, V. V. Simanovskaya, N. I. Il'chenko, and G. I. Golodets,Kinet. Katal.,23, No. 2, 398–401 (1982).
V. A. Roiter, G. I. Golodets, and Yu. I. Pyatnitskii (Pyatnitsky),Proceedings of the Fourth International Congress on Catalysis, Acad. Kiado, Budapest (1971), pp. 466–476.
N. I. Il'chenko and G. I. Golodets,J. Catal.,39, No. 1, 57–72, 73–86 (1975).
L. N. Raevskaya, F. G. Pavlovskii, B. I. Kolotusha, and N. I. Il'chenko,Ukr. Khim. Zh.,57, No. 10, 1080–1084 (1991).
L. N. Raevskaya, F. G. Pavlovskii, and N. I. Il'chenko,Ukr. Khim. Zh.,59, No. 10, 1040–1043 (1993).
N. I. Il'chenko, L. N. Raevskaya, A. I. Bostan, and G. I. Golodets,Kinet. Katal.,32, No. 4, 873–884 (1991).
N. I. Il'chenko,Teor. Éksp. Khim.,30, No. 3, 158–162 (1994).
D. J. Driscoll, W. Martir, J. X. Wang, J. H. Lunsford,J. Am. Chem. Soc.,107, 58–63 (1985).
C. H. Lin, K. D. Campbell, J. X. Wang, and J. H. Lunsford,J. Phys. Chem.,90, 534–537 (1986).
N. I. Il'chenko, N. V. Pavlenko, V. D. Pokhodenko, et al.,Eleventh International Congress on Catalysis, Baltimore, June 13 to July 5, 1996; Abstr., Rice University Print Center, Baltimore (1996), Poster-256.
Yu. I. Pyatnitskii, N. I. Il'chenko, and N. V. Pavlenko,Third Workshop “C 1 –C 3 Hydrocarbon Conversion” in memory of Prof. G. K. Boreskov, Krasnoyarsk, Russia, July 14–17, 1997; Book of Abstr. (1997), B7.
O. V. Krylov, M. U. Kislyuk, B. R. Shub, et al.,Kinet. Katal.,13, No. 3, 598–610 (1972).
N. I. Il'chenko and Yu. I. Pyatnitskii (Pyatnitsky),Second Workshop Meeting “C 1 –C 3 Hydrocarbon Conversion”, Krasnoyarsk, June 27–30, 1996; Abstr., Krasnoyarsk (1987), pp. 18–19.
Additional information
L. V. Pisarzhevskii Institute of Physical Chemistry, National Academy of Sciences of Ukraine, 31 Prospekt Nauki, Kiev 252039, Ukraine. Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 34, No. 5, pp. 265–281, September–October, 1998.
Rights and permissions
About this article
Cite this article
Il'chenko, N.I., Pyatnitskii, Y.I. & Pavlenko, N.V. Catalytic properties of the carbides of transition metals in oxidation reactions. Theor Exp Chem 34, 239–256 (1998). https://doi.org/10.1007/BF02523256
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02523256