Abstract
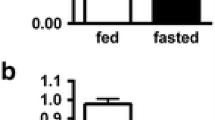
Preterm guinea pigs were delivered on day 65 of gestation (term=68 d) and were allowed either free or restricted access to food for the subsequent 48 h. Plasma phosphatidylcholine (PC) concentration increased postnatally from 190 (range 144–307) to 751 (426–1039) and 883 (758–977) μM for fed and starved pups, respectively. Plasma PC composition in both groups of pups was characterized by selective and equivalent relative increases to individual molecular species containing 18∶0 at thesn-1 position. Hepatic PC concentration increased from 6.75 (5.41–8.20) to 8.65 (6.54–10.63) and 9.23 (8.18–10.17) μmol/g for fed and starved pups, respectively, and, under all conditions, hepatic PC molecular composition closely mirrored that of plasma PC. These results support the hypothesis that the molecular species composition of plasma PC for the guinea pig in the immediate postnatal period is determined largely by the composition of the hepatic PC pool destined for lipoprotein secretion. Hepatic PC composition and concentration of the starved neonatal guinea pig were maintained independently of any dietary nutrient intake, at the expense of mobilization of extra hepatic lipid reserves. While this adaptive mechanism has inherent limited survival potential in neonatal starvation, it has implications for studies measuring plasma phospholipid fatty acid compositions as biochemical markers of dietary fat intake in preterm infants.
Similar content being viewed by others
Abbreviations
- EFA:
-
essential fatty acid
- HPLC:
-
high-performance liquid chromatography
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PUFA:
-
polyunsaturated fatty acid
- TFE:
-
trifluoroethanol
References
Innis, S.M. (1991) Essential Fatty Acids in Growth and Development,Prog. Lipid. Res. 30, 39–103.
Poisson, J.-P., Dupuy, J.P., Sarda, P., Descomps, B., Narce, M., Rieu, D., and de Paulet, A.C. (1993) Evidence That Liver Microsomes of Human Neonates Desaturate Essential Fatty Acids,Biochim. Biophys. Acta 1167, 109–113.
Carlson, S.E., Cooke, R.J., Werkman S.H., and Tolley E.A. (1992) First Year Growth of Preterm Infants Fed Standard Compared to Marine Oil n-3 Supplemented Formula,Lipids 27, 901–907.
Hoffman, D.R., and Uauy, R. (1992) Essentiality of Dietary ω3 Fatty Acids for Premature Infants: Plasma and Red Blood Cell Fatty Acid Composition,Lipids 27, 886–895.
Clandinin, M.T., Garg, M.L., Parrott, A., van Aerde, J., Hervada, A., and Lein, E. (1992) Addition of Long-Chain Polyunsaturated Fatty Acids to Formula for Very Low Birth Weight Infants,Lipids 27, 896–900.
Carlson, S.E., Wekman, S.H., Peeples, J.M., Cooke, R.J., and Tolley, E.A. (1993) Arachidonic Acid Status Correlates with First Year Growth in Preterm Infants,Proc. Natl. Acad. Sci. (USA) 90, 1073–1077.
Vance, J.E., and Vance, D.E. (1986) Specific Pools of Phospholipids Are Used for Lipoprotein Secretion by Cultured Rat Hepatocytes,J. Biol. Chem. 261, 4486–4491.
Yeagle, P.L. (1988) Lipid Regulation of Cell Membrane Structure and Function,FASEB J. 3, 1833–1842.
Burdge, G.C., Hunt, A.N. and Postle, A.D. (1994) Mechanisms of Hepatic Phosphatidylcholien Synthesis in Adult Rat: Effects of Pregnancy,Biochem. J. 303, 941–947.
Burdge, G.C., Kelly, K.J., and Postle, A.D. (1993) Mechanisms of Hepatic Phosphatidylcholine Synthesis in the Developing Guinea Pig: Contributions of Acyl Remodelling and ofN-Methylation of Phosphatidylethanolamine,Biochem. J. 290, 67–73.
Bøhmer, T., Havel, R.J., and Long, J.A. (1972) Physiological Fatty Liver and Hyperlipidemia in the Fetal Guinea-Pig: Chemical and Ultrastructural Characterization,J. Lipid Res. 13, 371–382.
Weaver, L.T., and Lucas, A. (1987) Upper Intestinal Mucosal Proliferation in the Newborn Guinea Pig: Effect of Composition of Milk Feeds,Pediatr. Res. 22, 675–678.
Stanley, C.A., Gonzales, E., and Baker, L. (1983) Development of Hepatic Fatty Acid Oxidation and Ketogenesis in the Newborn Guinea Pig,Pediatr. Res. 17, 224–229.
Burdge, G.C., and Postle, A.D. (1994) Hepatic Phospholipid Molecular Species in the Guinea Pig: Adaptations to Pregnancy,Lipids 29, 259–264.
Bligh, E.G., and Dyer, W.S. (1959) A Rapid Method for Total Lipid Extraction and Purification,Can. J. Biochem. 37, 911–923.
Burdge, G.C., and Postle, A.D. (1995) Effect of Maternal Ethanol Consumption During Pregnancy on the Phospholipid Molecular Species Composition of Fetal Guinea-Pig Brain, Liver and Plasma,Biochim. Biophys. Acta 1256, 346–352.
Postle, A.D. (1987) A Method for the Sensitive Analysis of Individual Molecular Species of Phosphatidylcholine by High Performance Liquid Chromatography with Post-Column Fluorescence Detection,J. Chromatogr. 415, 41–51.
Burdge, G.C., and Postle, A.D. (1995) Phospholipid Molecular Species Composition of Developing Fetal Guinea Pig Brain,Lipids 30, 719–724.
Keppler, D., and Decker, K. (1984) Glycogen, inBergmeyer: Methods of Enzymatic Analysis (Bergmyer, J., and Grassi, M., eds.) Vol. 6, pp. 11–18, Verlag Chemie, Weinheim.
Foote, K.D., MacKinnon, M.J., and Innis, S.M. (1991) Effect of Early Introduction of Formula vs. Fat-Free Parenteral Nutrition on Essential Fatty Acid Status of Preterm Infants,Am. J. Clin. Nutr. 54, 93–97.
Postle, A.D., Al, M.D.M., Burdge, G.C., and Hornstra, G. (1995) The Composition of Individual Molecular Species of Plasma Phosphatidylcholine in Human Pregnancy,Early Human Devel. 43, 47–58.
Lyman, R.L., Tinoco, J., Bouchard, P., Sheenan, G., Ostwald, R., and Miljanich, P. (1967) Sex Differences in the Metabolism of Phosphatidylcholines in Rat Liver,Biochim. Biophys. Acta 137, 107–114.
Dauprat, P., Aurousseau, B., Bauchart, D., Dalle, M., and Delost, P. (1985) Influence of Psychosomatic Stress in Pregnant Guinea-Pigs on Fetal Lipid Metabolism,J. Devel. Physiol. 7, 339–345.
Foreman-van Drongelen, M.M.P.H., Houwelingen, A.C.V., Kester, A.D.M., de Jong, A.E.P., Blanco, C.E., Hasaart, T.H.M., and Hornstra, G. (1995) Long-Chain Polyene Status of Preterm Infants with Regard to the Plasma Fatty Acid Composition of Their Diet: Comparison Between Absolute and Relative Fatty Acid Levels in Plasma and Erythrocyte Phospholipids,Br. J. Nutr. 73, 405–422.
Neuringer, M., and Connor, W.E. (1986) n-3 Fatty Acids in the Brain and Retina; Evidence for Their Essentiality,Nutr. Rev. 44, 285–294.
Clandinin, M.T., Chappell, J.E., Leong, S., Heim, T., Swyer, P.R., and Chance, S.W. (1980) Intrauterine Fatty Acid Accretion Rates in Human Brain: Implications for Fatty Acid Requirements,Early Human Devel. 4, 121–129.
Clandinin, M.T., Chappell, J.E., Leong, S., Heim, T., Swyer, P.R., and Chance, S.W. (1980) Extrauterine Fatty Acid Accretion Rates in Human Brain: Implications for Fatty Acid Requirements,Early Human Devel. 4, 131–138.
Dobbing, J., and Sands, J. (1979) The Quantitative Growth and Development of Human Brain,Early Human Devel. 3, 79–138.
Sinclair, A.J., and Crawford, M.A. (1972) The Accumulation of Arachidonate and Docosahexaenoate in the Developing Rat Brain,J. Neurochem. 19, 1753–1758.
Shires, S.E., Conway, S.P., Rawson, I., Dear, P.R.F., and Kelleher, J. (1986) Fatty Acid Composition of Plasma and Erythrocyte Phospholipids in Preterm Infants,Early Human Devel. 13, 53–63.
Hunt, A.N., Bellhouse, A.F., Kelly, F. J., and Postle, A.D. (1991) Late Gestation Changes in Rat Tissue Phosphatidylcholine Composition,Biochem. Soc. Trans. 19, 111S.
Altman, J., and Das, G.D. (1967) Postnatal Neurogenesis in the Guinea-Pig,Nature 214, 1089–1101.
Author information
Authors and Affiliations
About this article
Cite this article
Hunt, A.N., Burdge, G.C. & Postle, A.D. Phospholipid composition of neonatal guinea pig liver and plasma: Effect of postnatal food restriction. Lipids 31, 489–495 (1996). https://doi.org/10.1007/BF02522642
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02522642