Abstract
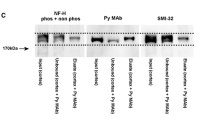
The Tau-1 monoclonal antibody was localized to the nucleolus of interphase cells and the nucleolar organizing regions (NORs) of acrocentric chromosomes in cultured human cells. Putative nucleolar and NOR tau was found in HeLa cells and lymphoblasts as well as in nontransformed fibroblasts and lymphocytes. To confirm the presence of tau in the nuclei of these nonneural cells, immunoblotting analysis was performed on isolated nuclei from lymphoblasts. Several tau bands were noted on the blot of the nuclear extract suggesting the presence of multiple tau isoforms. Tau-1 immunostaining demonstrated variable staining intensities between individual acrocentric chromosomes in all cells tested. In cultured peripheral lymphocytes, these staining patterns were the same from one chromosome spread to the next within an individual. This consistency of Tau-1 staining and its variability among NORs was reminiscent of staining patterns obtained using the silver-NOR procedure. Comparisons of Tau-1 immunostaining with silver staining of chromosome spreads from human lymphocytes demonstrated that Tau-1 did not immunostain all of the NORs that were silver stained. The intensity of Tau-1 fluorescence in nucleoli was further shown to be increased in phytohemagglutinin-stimulated lymphocytes, indicating an upregulation of nuclear tau when cells reentered the cell cycle. These results contribute to a growing body of evidence defining tau as a multifunctional protein that may be involved in ribosomal biogenesis and/or rRNA transcription in the nucleus of all cells as well as microtubule-stabilizing functions in the neuronal cytoplasm.
Similar content being viewed by others
References
Ashman JB, Hall ES, Eveleth J, Boekelheide K (1992) Tau, the neuronal heat-stable microtubule-associated protein, is also present in the cross-linked microtubule network of the testicular spermatid manchette. Biol Reprod 46:120–129
Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J. Cell Biol 101:1371–1378
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brady RM, Zinkowski RP, Binder LI (1995) Presence of tau in isolated nuclei from human brain. Neurobiol Aging 16:479–486
Brinkley BR, Zinkowski RP, Mollon WL, Davis RM, Pisegna MA, Pershouse M, Rao RM (1988) Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature 336:251–254
Caizergues-Ferrer M, Mariottinin P, Curie C, Lapeyre B, Cas N, Amalric F, Amaldi F (1989) Nucleolin fromXenopus laevis: cDNA cloning and expression during development. Genes Dev 3:324–333
Drubin DG, Kirschner MW (1986), Tau proteins function in living cells. J Cell Biol 103:2739–2746
Goedert M, Wischik CM, Crowther FA, Walker JE, Klug A (1988) Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer's disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA 85:4051–4055
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3:519–526
Goodpasture S, Bloom SE (1975) Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53:37
Greenberg SG, Davies P, Schein JD, Binder LI (1992) Hydrofluoric acid-treated tau-PHF proteins display the same biochemical properties as normal tau. J Biol Chem 267:564–569
Grundke-Iqbal I, Iqbal K, Tung Y, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917
Henderson AS, Warburton D, Atwood KC (1972) Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci USA 69:3394–3398
Himmler A (1989) Structure of bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol 9:1389–1396
Himmer A, Drechsel D, Kirschner WK, Martin DW (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol 9:1381–1388
Howell WM (1982) Selective staining of Nucleolus Organizing Regions (NORs). In: Busch H, Rothblum L (eds) The cell nucleus, vol XI. Academic Press, New York, pp 89–142
Howell WM, Denton TE, Diamond JR (1975) Differential staining of the satellite regions of human acrocentric chromosomes. Experimentia 31:260–262
Ihara Y, Abraham C, Selkoe DJ (1983) Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature 304:727–730
Iqbal K, Zaidi T, Thompson CH, Mertz PA, Wisniewski HM (1984) Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol 62:167–177
Jordan G (1987) At the heart of the nucleolus. Nature 329:489–490
Kosik KS, Orechio LD, Binder LI, Trojanowski JQ, Lee VM, Lee G (1988) Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1:817–825
Ksiezak-Reding H, Binder LI, Yen SH (1990) Alzheimer's disease proteins (A68) share epitopes with tau but show distinct biochemical properties. J Neurosci Res 25:420–430
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee G, Cowan N, Kirschner MW (1988) The primary structure and heterogeneity of tau protein from mouse brain. Science 239:285–288
Lichtenberg BE, Mandelkow M, Hagestadt T, Mandelkow E (1988) Structure and elasticity of microtubule-associated protein tau. Nature 34:359–362
Litman P, Barg J, Rindzoonski L, Ginzburg I (1993) Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron 10:627–638
Liu W-K, Moore WT, Williams RT, Hall FL, Yen SH (1993) Application of synthetic phospho- and unphospho-peptides to identify phosphorylation site in a subregion of the tau molecule, which is modified in Alzheimer's disease. J Neurosci Res 34:371–376
Loomis PA, Howard TH, Castleberry RP, Binder LI (1990) Identification of nuclearl isoforms in human neuroblastoma cells. Proc Natl Acad Sci USA 87:8422–8426
LoPresti P, Szuchet S, Papasozomenos SCh, Zinkowski RP, Binder LI (1995) Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci USA 92:10369–10373
Lu Q, Wood JG (1993) Characterization of fluorescently derivatized bovine tau protein and its localization and functions in cultured chinese hamster ovary cells. Cell Motil Cytoskeleton 25:190–200
Mamrack MD, Olsen MOJ, Busch H (1979) Amino acid sequence and sites of phosphorylation in a high acidic region of nucleolar nonhistone protein C23. Biochemistry 18:3381–3386
Markovic VD, Worton RG, Berg JM (1978) Evidence for the inheritance of silver-stained nucleolus organizer regions. Hum Genet 41:181
Metuzals J, Robitaille Y, Houghton S, Gauthier S, Leblanc R (1988) Paired helical filaments and the cytoplasmic-nuclear interface in Alzheimer's disease. J Neurocytol 17:827–833
Migheli A, Brown M, Brown K, Shelanski ML (1988) Light and electron microscope localization of the microtubule-associated tau protein in rat brain. J Neurosci 8:1846–1851
Mitchison TJ, Kirschner MW (1985) Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol 101:755–765
Papasozomenos SCh (1989) Tau protein immunoreactivity in dementia of the Alzheimer type: II. Electron microscopy and pathogenic implications. Effects of fixation on the morphology of Alzheimer's abnormal filaments. Lab Invest 60:375–388
Papasozomenos SCh (1991) Association of tau with ribosomes in Alzheimer's disease. Soc Neurosci Abst 17:1069
Papasozomenos SCh (1995) Nuclear tau immunoreactivity in presenile dementia with motor neuron disease: a case report. Clin Neuropathol 14:100–104
Papasozomenos SCh, Binder LI (1987) Phosphorylation determines two distinct species of tau in the central nervous system. Cell Motil Cytoskeleton 8:210–226
Rooney DE, Czepulkowski BH (1986) Human genetics: a practical approach. IRL Press, Oxford, p 41
Sadot E, Marx R, Barg J, Behar L, Ginzburg I (1994) Complete sequence of 3′-untranslated region of tau from rat central nervous system: implications for mRNA heterogeneity. J Mol Biol 241:325–331
Shi Y, Thomas JO (1992) The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognatel. Mol Cell Biol 12:2186–2192
Sommerville J (1986) Nucleolar structure and ribosome biogenesis. Trends Biol Sci 11:438–442
Towbin H, Staehlin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USa 76:4354–4356
Wang Y, Loomis PA, Zinkowski RP, Binder LI (1993) A novel tau transcript in cultured human neuroblastoma cells expressing nuclear tau. J Cell Biol 121:257–267
Welch JW, Mizzen LA (1988) Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol 106:1117–1130
Wischi CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA (1988) Structural characterization of the core of the paired helical filament of Alzheimer's disease. Proc Natl Acad Sci USA 85:4884–4888
Author information
Authors and Affiliations
Additional information
J. B. Rattner
Rights and permissions
About this article
Cite this article
Thurston, V.C., Zinkowski, R.P. & Binder, L.I. Tau as a nucleolar protein in human nonneural cells in vitro and in vivo. Chromosoma 105, 20–30 (1996). https://doi.org/10.1007/BF02510035
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02510035