Summary
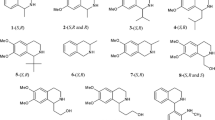
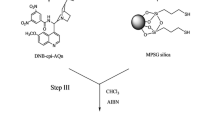
Six new quinine (QN) O9-hydrazide derivatives with different substituents have been synthesized and immobilized on porous silica gel for HPLC. The chiral resolving power of these anion-exchange-type chiral stationary phases (CSPs) has been investigated and compared with that of four carbamate QN derivatives with analogous substitution. The unsubstituted QN-hydrazide derivative was usually the best chiral selector of the hydrazide series. Among the substituted hydrazide derivatives the introduction of a tritylcarbonyl or atert-butylcarbonyl group at the β position of the hydrazide function improved chiral recognition by the resulting CSPs. Although carbamate functionality seemed to favour the resolution of the enantiomers of many of the racemic compounds tested, the hydrazide series resulted in better separations of the enantiomers of the DNP derivatives of amino acids and of certain acidic drugs of therapeutic interest, such as the profens. The selectivity factors of these types of compounds on these QN-hydrazide derivatives are the best yet obtained on QN-derived chiral selectors.
Similar content being viewed by others
References
S. C. Stinson, Chem. Ind.21, 83 (1998).
W. Lindner, M. Lämmerhofer, Eur. Pat. Appl. No. 96 109 072. 7.
M. Lämmerhofer, W. Lindner, J. Chromatogr. A741, 33 (1996).
W. Lindner, M. Lämmerhofer, GIT Special-Chromatography International96, 16 (1996).
O. P. Kleidernigg, M. Lämmerhofer, W. Lindner, Enantiomer1, 387 (1996).
V. Piette, M. Lämmerhofer, K. Bischoff, W. Lindner, Chirality9, 157 (1997).
M. Lämmerhofer, N. M. Maier, W. Lindner, Am. Lab.30, 71 (1998).
N. M. Maier, L. Nicoletti, M. Lämmerhofer, W. Lindner, Chirality11, 522 (1999).
M. Lämmerhofer, W. Lindner, J. Chromatogr. A829, 115 (1998).
P. Franco, M. Lämmerhofer, P. M. Klaus, W. Lindner, J. Chromatogr. A, in press (1999).
E. Veigl, B. Böhs, A. Mandl, D. Krametter, W. Lindner, J. Chromatogr. A645, 151 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Franco, P., Lämmerhofer, M., Klaus, P.M. et al. Novel cinchona alkaloid O9-hydrazide derivatives as chiral anion-exchange-type selectors: Synthesis and chromatographic evaluation. Chromatographia 51, 139–146 (2000). https://doi.org/10.1007/BF02490555
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02490555