Abstract
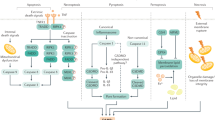
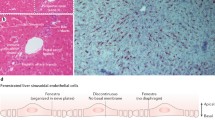
This report reviews studies addressing the new concepts in liver regeneration after a partial hepatectomy (PHx). The review begins with an overview of the immunologic mechanisms of liver regeneration after PHx, especially regarding Kupffer cells and extrathymic T cells of the regenerative liver in the cell-mediated immunity, based on major histocompatibility complex I and II antigens. Attention is then devoted to “on and off” studies in liver regeneration after PHx, by hypothesizing the shear stress based on the fact that the portal flow against hepatocytes or sinusoidal endothelial cells triggers their regeneration after a partial hepatectomy and controls the volume of the regenerating liver by the stimulating the cell surface modulator (CSM) of hepatocytes and sinusoidal endothelial cells (SEC). We propose that the acute elevation of shear stress after PHx influences the adhesion between SEC and intrahepatic leukocytes. These concepts are expected to positively contribute to the future research on liver regeneration after PHx.
Similar content being viewed by others
References
Minoru O, Noriaki T, Kunzo O (1984) Activation of NK activity and auto-reactive cytotoxicity after hepatectomy. Acta Med Okayama 38:207–213
Flye MW, Yu S (1990) Augmentation of cell-mediated cytotoxicity following 50% partial hepatectomy. Transplantation 49:581–587
Miyahara S, Yokomuro K, Takahashi H, Kimura Y (1983) Regeneration and the immune system I. In vitro and in vivo activation of lymphocytes by liver regeneration and the role of Kupffer cells in stimulation. Eur J Immunol 13:878–883
Pinto M, Herzberg H, Barnea A, Shenberg E (1987) Effects of partial hepatectomy on the immune response in mice. Clin Immunol Immunopathol 142:123–132
Sato Y, Inoue S, Nagao T, Yoshida K, Akiyama N, Muto T (1991) Cyclosporine suppresses class II antigen expression in regenerating liver of rats after partial hepatectomy. Jpn Gastroenterol Surg 24:172
Sato Y, Tsukada K, Yoshida K, Muto T, Matsumoto Y (1992) FK506 suppresses class II antigen expression in regenerating livers following partial hepatectomy in the rat. Transplant Proc 24:1628–1630
Sato Y, Tsukada K, Matsumoto Y, Abo T (1993) Interferon-γ inhibits liver regeneration by stimulating major histocompatibility complex class II antigen expression by regenerating liver. Hepatology 18:340–346
Sato Y, Farges O, Delphine B, Bismuth H (1995) Intrahepatic lymphocytes following 70% partial hepatectomy in the rats: extrathymic T cells in the rats. Int Hepatol Commun 3 suppl:S132
Sato Y, Farges O, Delphine B, Bismuth H (1996) Mechanism of extrathymic and thymic T cells following 70% PHx in the rats. Hepatology 21(1):I-74
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173
Guy-Grand D, Cerf-Bensussan N, Briottet C (1991) Two gut intraepithelial CD8+ lymphocyte populations with different T-cell receptors: a role for the gut epithelium in T-cell differentiation. J Exp Med 173:471–481
Abo T, Ohteki T, Seki S, Koyamada N, Yoshikai Y, Masuda T, Rikiishi H, Kumagai K (1991) The appearance of T cells bearing self-reactive T-cell receptors in the livers of mice injected with bacteria. J Exp Med 174:417–424
Seki S, Abo T, Sugiura K, Kumagai K (1991) Unusual αβ T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol 147:1214–1221
Sato Y, Tsukada K, Iiai T, Ohmori K, Yoshida K, Muto T, Watanabe Y, Matsumoto Y, Abo T (1993) Activation of extrathymic T cells in the liver during liver regeneration following partial hepatectomy. Immunology 78:86–91
Sato Y, Farges O, Delphine B, Bismuth H (1998) Detection of intermediate TcR cells, NK3.2.3-positive T cells, and CD5+ B cells in older rats. Acta Med Biol (in press)
Itoh H, Abo T, Sugawara S, Kanno A, Kumagai K (1988) Agerelated variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunol 141:315–323
Abo T, Ohteki T, Seki S, Koyamada N, Yoshikai Y, Masuda T, Rikiishi H, Kumagai K (1991) The appearance of T cells bearing self-reactive T-cell receptors in the livers of mice injected with bacteria. J Exp Med 174:417–424
Fausto N (1994) Liver stem cells In: Arias IM, Boyer JL, Fausto N, Jokoby WB, Schachter D, Shafritz DA (eds) The liver: biology and pathology, 3rd edn. Raven Press New York, pp 1501–1518
Sato Y, Koyama S, Tsukada K, Hatakeyama K (1997) Acute portal hypertension reflecting shear stress is a trigger of liver regeneration following partial hepatectomy. Surgery Today 27 (in press)
Kamiya A, Togawa T (1980) Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol 239:H14-H21
Ando J, Tsuboi H, Korenaga R, Takada Y, Toyama SN, Miyasaka M, Kamiya A (1994) Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by downregulating VCAM-1 expression. Am J Physiol 267:C679-C687
Hsieh HJ, Li NQ (1993) Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol 154:143–151
Ohno M, Lopez F, Gibbons GH, Cooke JP, Dzau VJ (1992) Shear stress induced TGF-beta 1 gene expression of active TGF-beta 1 is mediated via a K+ channel (abstract). Circulation 86:I-87
Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT (1993) Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 268:17478–17488
Morello D, FitzGerald MJ, Babinet C, Fausto N (1990) c-myc, c-fos, and c-jun regulation in the regenerating livers of normal and H2K/c-myc transgenic mice. Mol Cell Biol 10:3185–3193
Fausto N, Mead JE, Gruppuso PA, Braun L (1990) TGF-beta in the liver development, regulation and carcinogenesis. Ann NY Acad Sci 593:231–242
Michalopoulos GK (1990) Liver regeneration: molecular mechanisms of growth control. FASEB J 4:176–187
Sonsoles H, Beatrice D, Ana MG, Maria JMD-G, Lisardo B (1995) Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology 21:776–786
Braet F, Zanger RD, Baekeland M, Crabbe E, Van Der Smissen P, Wisse E (1995) Structure and dynamics of the fenestrae-associated cytoskeleton of rat liver sinusoidal endothelial cells. Hepatology 21:180–189
Whitfield JF, Boynton AL, Rixon RH (1985) The control of cell proliferation by calcium, Ca2+ calmodulin, and cyclic AMP. In: Boynton AL, Leffert HN (eds) Control of animal cell proliferation. Academic, New York, pp 331–365
Koch KS, Leffert HN (1979) Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell 18:153–163
Yee AG, Revel JP (1978) Loss and reappearance of gap junctions in regenerating liver. J Cell Biol 78:554–564
Schaffner F, Popper H (1963) Capillarization of hepatic sinusoid in man. Gastroenterology 44:239–242
Fraser R, Bowler LM, Day WA, Dobbs B, Johnson HD, Lee D (1980) High perfusion pressure damages the sieving ability of sinusoidal endothelium in rat livers. Br J Exp Pathol 61:222–228
Oda M, Nakamura M, Watanabe N, Ohya Y, Sekuzuka E, Tsukada N, Yonei Y (1983) Some dynamic aspects of the hepatic microcirculation: demonstration of sinusoidal endothelail fenestrae as a possible regulatory factor. In: Tsuchiya M, Wayland H, Oda M, Okazaki I (eds) Intravital observation of organ microcirculation. Excerpta Medica, Amsterdam, pp 105–138
Nakata K (1961) Direct measurement of blood pressures in minute vessels of the liver. Am J Physiol 199:1181–1188
Rous P, Larimore LD (1920) Relation of the portal blood to liver maintenance. J Exp Med 31:609–632
Mann FC, Fishback FC, Gay JG, Green GF (1931) Experimental pathology of the liver; Studies III, IV, V. Arch Pathol (Chicago) 12:787–807
Bollman JL (1961) The animal with an Eck fistula. Physiol Rev 41:607–621
Fisher B, Russ C, Updegraff H, Fisher ER (1954) Effect of increased hepatic blood flow upon liver regeneration. Arch Surg 69:263–272
Clarke AM, Thomson RY, Fraenkel GJ (1968) Vascular factors in liver regeneration. SGO 126:45–52
Bucher NLR, Swaffield MN (1964) The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res 24:1611–1625
Starzl TE, Porter KA, Putnam CW (1975) Intraportal insulin protects from the liver injury of portacaval shunt in dogs. Lancet 2:1241–1246
Francavilla A, Ove P, Wu SK, DiLeo A, Van Thiel D, Starzl TE (1982) Extraction of hepatic stimulatory activity (HSA) from (adult) rat liver following T3 injection (abstract). Hepatology 2:704
Francavilla A, Barone M, Van Thiel DH, Mazzaferro V, Prelich J, Starzl TE (1991) Further steps of HSS (hepatic stimulatory substance) purification. Dig Dis Sci 36:674–680
Kan M, Huang J, Mansson PE, Yasumitsu H, Carr B, McKeehan WL (1989) Heparin-binding growth factor type 1 (acidic fibroblast growth factor): a potential biphasic autocrine and paracrine regulator of hepatocyte regeneration. Proc Natl Acad Sci USA 86:7432–7436
Marsden ER, Hu Z, Fujio K, Nakatsukasa H, Thorgeirsson SS, Evarts RP (1992) Expression of acidic fibroblast growth factor in regenerating liver and during hepatic differentiation. Lab Invest 67:427–433
Cruise JL, Houck KA, Michalopouls G (1985) Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha-1 adrenoreceptor by norepinephrine. Science 227:749–751
Russell WE, Bucher NLR (1983) Vasopressin modulates liver regeneration in the Brattleboro rat. Am J Physiol 245:G321-G324
MacManus JP, Braceland BM (1976) A connection between the production of prostaglandins during liver regeneration and the DNA synthesis responses. Prostaglandins 11:609–620
McGowan JA, Strain AJ, Bucher NL (1981) DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol 108:353–363
Skov Olsen P, Boesby S, Kirkegaard P, Therkelsen AT, Poulsen SS, Nexo E (1988) Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology 8:922–996
Mead JE, Fausto N (1989) Transforming growth factor α may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA 86:1558–1562
Russell WE, Dempsey PJ, Sitaric C, Peck AJ, Coffey RJ Jr (1993) Transforming growth factor-alpha (TGF-α) concentrations increase in regenerating rat liver: evidence for a delayed accumulation of mature TGF alpha. Endocrinology 133:1731–1738
Russell WE, McGowan JA, Bucher NLR (1984) Biological properties of a hepatocyte growth factor from rat platelets. J Cell Physiol 119:193–197
Webber EM, FitzGerald MJ, Brown PI, Bartlett MH, Fausto N (1993) TGF-α expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between TGF-α and HGF. Hepatology 18:1422–1431
Lindroos PM, Zarnegar R, Michalopoulos GK (1991) Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 13:743–749
Fausto N, Webber EM (1993) Control of liver growth. Crit Rev Eukaryot Gene Express 3:117–135
Fausto N, Webber EM (1993) Mechanisms of growth regulation in liver regeneration and hepatic carcinogenesis. In: Boyer JL, Ockner K (eds) Progress in liver disease XI. WB Saunders, Philadelphia
Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM (1992) Antibodies to tumor necrosis factor-α inhibit liver regeneration after partial hepatectomy. Am J Physiol 263:G579-G585
Schreck R, Rieber P, Baeuerie PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of NF-kB transcription factor and HIV-1. EMBO J 10:2247–2258
Banerjee R, Karpen S, Siekevitz M, Lengyel G, Bauer J, Acs G (1989) Tumor necrosis factor-α induces a kappa B sequence-specific DNA-binding protein in human hepatoblastoma HepG2 cells. Hepatology 10:1008–1013
Mito M, Ackroyed FW, Covelli VH, Eyskens E, Katayama I, McDermott WV (1967) Partial heterotopic liver homograft in dogs utilizing portal arterialization. Ann Surg 165:20–32
Child CG, Barr D, Holswade GR, Harrison CS (1953) Liver regeneration following portacaval transposition in dogs. Ann Surg 138:600–608
Sigel B, Baldia LB, Brightman SA, Dunn MR (1968) Effect of blood flow reversal in liver autotransplants upon the site of hepatocyte regeneration. J Clin Invest 47:1231–1237
Isomura H, Sawada N, Nakajima Y, Sakamoto H, Ideda T, Kijima T, Enomoto K, Mori M (1993) Increase in portal flow induces c-myc expression in isolated perfused rat liver. J Cell Physiol 154:329–332
Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CFJ, Gimbrone MA (1993) Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA 90:4591–4595
Bucher EC (1991) Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 67:1033–1036
Lawrence MB, Springer TA (1991) Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859–873
Tanaka Y, Adams DH, Shaw S (1993) Proteoglycan on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today 14:111–115
Nagel T, Resnick N, Atkinson WJ, Dewey CF, Gimbrone MA Jr (1994) Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest 94:885–891
Lawrence MB, McIntire LV, Eskin SG (1994) Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Am J Physiol 267:1284–1290
Chen S, Alon R, Fuhlbrigge RC, Springer TA (1997) Rolling and transient tethering of leukocytes on antibodies reveal specializations of selections. Proc Natl Acad Sci USA 94:3172–3177
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sato, Y., Tsukada, K. & Hatakeyama, K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today 29, 1–9 (1999). https://doi.org/10.1007/BF02482962
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02482962