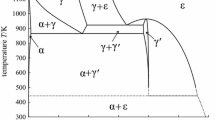
Abstract
The Fe−C system has been studied quite thoroughly. However, some aspects of the system remain underdeveloped or at least disputable. This refers especially to the carbide part of the diagram. There is no unanimous opinion on the nature of melting of cementite (congruent or incongruent) and its melting temperature. Light carbide phases other than cementite are not represented by the diagram and there is no correspondence between the low-temperature part of the diagram and existing experimental data on carbide transformations in tempering of quenched steel.
Similar content being viewed by others
References
M. Hellert, “Solubility of cementite in liquid iron,”Acta Metallurgica,3(1), 37 (1955).
A. A. Zhukov,Geometric Thermodynamics of Iron Alloys [in Russian], Metallurgiya, Moscow (1979).
A. A. Zhukov, L. E. Shterenberg, and V. A. Shalashov, “Pseudohexagonal iron carbide Fe7C3 and the Fe3C−Fe7C3 eutectic in the Fe−C system,”Izv. Akad. Nauk SSSR, Metally, No. 1, 181–184 (1973).
H. C. Eckstrom and W. A. Adcock, “New iron carbide in hydrocarbon synthesis catalysis,”J. Amer. Ceram. Soc.,72, 1042 (1950).
G. V. Samsonov and I. M. Vinitskii,Refractory Compounds [in Russian], Metallurgiya, Moscow (1976).
A. A. Zhukov et al., “Phase diagram of the iron-diamond system,” in:Structure of Phases. Phase Transformations, and Phase Diagrams of Metallic Systems, Coll. of Works [in Russian], Nauka, Moscow (1974), pp. 99–103.
A. A. Zhukov and R. L. Snezhnoi, “On the metastable iron-carbon system,”Izv. Akad. Nauk SSSR, Metally, No.4, 209–212 (1973).
H. Schenck, E. Steinmetz, and M. Gloz, “Die thermodynamischen Eigenschaften des Systems Eisenkohlenstoff der Kohlenstoffrehen Seite des Systems,”Archiv Eisenhüttenwesen,42(5), 307 (1971).
A. A. Zhukov and L. N. Snezhnoi, “On the shape of the liquidus curve in the region of cementite melting in the iron-diamond phase diagram,”Izv. Akad. Nauk SSSR, Metally, No. 3, 192–199 (1976).
I. A. Korsunskaya, D. S. Kamenskaya, and T. P. Ershova, “A full T−P−C-phase diagram of the Fe−C system below a pressure of 50 kbar with allowance for the equilibria with cementite and diamond,” in:Common Features in the Structure of Phase Diagrams of Metallic Systems. Coll. of Works [in Russian], Nauka, Moscow (1973), pp. 66–75.
V. K. Grigorovich,The Electron Structure and Thermodynamics of Iron Alloys [in Russian], Nauka, Moscow (1970).
B. M. Mogutnov, I. A. Tomilin, and L. A. Shvartsman,The Thermodynamics of Iron-Carbon Alloys [in Russian], Metallurgiya, Moscow (1972).
G. I. Sil'man, “Use of the theory of regular solutions to recalculate the thermodynamic activity of carbon in austenite for different standard states,” in:Thermodynamics, Physical Kinetics of Structure Formation, and Properties of Cast Iron and Steel, Coll. of Works, Issue 4 [in Russian], Metallurgiya, Moscow (1971), pp. 58–60.
G. I. Sil'man, “To the problem of the choice of standard state of the components of multiphase systems,”Izv. Vuzov. Chern. Metal., No. 7, 8–13 (1976).
L. S. Darken and R. V. Gurry,The Physical Chemistry of Metals [Russian translation], Metallurgiya, Moscow (1960).
G. I. Sil'man, “A method for calculating phase diagrams of ternary systems with the use of coefficients of the interphase distribution of elements. Part 1: Biphase equilibrium,”Zh. Fiz. Khim.,57(2), 307–313 (1983); “Part 2. Three-phase and four-phase equilibria,”Zh. Fiz. Khim.,57(3), 548–554 (1983).
The Metallography of Iron. Vol. I: Foundations of Metallography [Russian translation], Metallurgiya, Moscow (1972).
Taiji Nishizawa and Bjorn Uhrenius, “A thermodynamic study of the Fe−Cr−C system at 1000°C,”Scand. J. Met.,6(2), 67–73 (1977).
H. Brandis, H. Preisendanz, and P. Schüller, “Untersuchungen über den Einflub der Legierungenselemente Cr, Mo, und W auf die Aktivität des Kohlenstoffes in Fe−X−C-Legierungen im Temperaturbereich von 900 bis 1100°C,”Thyssen Edelstahl Techn. Ber.,6(2), 155–167 (1980).
G. I. Sil'man, “Estimating the mutual influence of the components of a ternary system on the thermodynamic activities in the biphase region,”Zh. Fiz. Khim.,51(5), 1044–1047 (1977).
Author information
Authors and Affiliations
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 11, pp. 2–7, November, 1997.
Many concepts of the present paper contradict generally accepted notions. We invite the readers to take part in the discussion of the problem.
Rights and permissions
About this article
Cite this article
Sil'man, G.I. Refinement of the Fe−C diagram on the basis of results of a thermodynamic analysis and generalization of data for Fe−C and Fe−C−Cr systems. Met Sci Heat Treat 39, 451–456 (1997). https://doi.org/10.1007/BF02469111
Issue Date:
DOI: https://doi.org/10.1007/BF02469111