Abstract
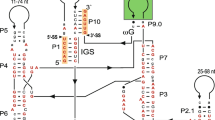
An insertion sequence was detected near the 3′ end of the nuclear small subunit rDNA in isolates ofPhialophora gregata f. sp.adzukicola, the causal agent of the brown stem rot disease of adzuki bean. This insertion sequence was absent in isolates ofP. gregata, f. sp.sojae which causes brown stem rot of soybean. The insertion sequence is 304 bp long and contains all the characteristics of group I introns. These characteristics include, the four conserved sequence elements (P, Q, R, and S), a U at the 5′ splice site of the exon, a G at the 3′ splice site of the intron, a putative internal guiding sequences; the sequence also fits a secondary structure model for group I introns. Similar to most group I introns found in nuclear small subunit rDNA, the intron was located in a highly conserved region and is devoid of long open reading frames. This intron provides a convenient marker for use in conventional PCR to separateP. gregata f. sp.adzukicola fromP. gregata f. sp.sojae.
Similar content being viewed by others
Literature cited
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. and Lipman, D. J. 1990. Basic local alignment search tool. J. Mol. Biol.215: 403–410.
Arnheim, N., Krystal, M., Schmickel, R., Wilson, G., Ryder, O. and Zimmer, E. 1980. Molecular evidence for genetic exchange among ribosomal genes on nonhomologous chromosomes in man and apes. Proc. Natl. Acad. Sci. USA77: 7323–7327.
Burke, J. M., Belfort, M., Cech, T. R., Davies, R. W., Schweyen, R. J., Shub, D. A., Szostak, J. W. and Tabak, H. F. 1987. Structural conventions for group I introns. Nucleic Acids Res.15: 7217–7221.
Cech, T. R. 1990. Self-splicing of group I introns. Ann. Rev. Biochem.59: 543–568.
Chen, W., Gray, L. E. and Grau, C. R. 1996a. Molecular differentiation of fungi associated with brown stem, rot and detection ofPhialophora gregata in resistant and susceptible soybean cultivars. Phytopathology86: 1140–1148.
Chen, W., Shearer, C. A. and Crane, J. L. 1996b. Identification of a group I intron in the nuclear small subunit rDNA ofPseudohalonectria lignicola. Current Microbiology33: 84–88.
DePriest, P. T. 1993. Small subunit rDNA variation in a population of lichen fungi due to optional group-I introns. Gene134: 67–74.
Dover, G. and Coen, E. 1981. Springcleaning ribosomal DNA: a model for multigene evolution? Nature290: 731–732.
Dujon, B. 1989. Group I introns as mobile genetic elements: facts and mechanistic speculations—a review. Gene82: 91–114.
Gargas, A., DePriest, P. T. and Taylor, J. W. 1995. Positions of multiple insertions in SSU rDNA of lichen-forming fungi. Mol. Biol. Evol.12: 208–218.
Gilbert, D. G. 1992. LoopDLoop, A Macintosh program for drawing RNA secondary structure. Published electronically on the Internet, avaiable, via anonymous ftp to ftp. bio. indiana.edu.
Gray, L. E. and Hepburn, A. G. 1992. Mitochondrial DNA restriction patterns ofPhialophora gregata isolates from soybean and adzuki bean. Phytopathology82: 211–215.
Gray, L. E. and Pataky, J. K. 1994. Reaction of mung bean plants to infection by isolates ofPhialophora gregata. Plant Dis.78: 782–785.
Jaeger, J. A., Turner, D. H. and Zuker, M. 1989. Improved predictions of secondary structures for RNA. Proc. Natl. Acad. Sci. USA86: 7706–7710.
Jaeger, J. A., Turner, D. H. and Zuker, M. 1990. Predicting optimal and suboptimal secondary structure for RNA. Methods in Enzymology183: 281–306.
Kobayashi, K., Kondo, N., Ui, T., Tachibana, H. and Aota, T. 1983. Difference in pathogenicity ofPhialophora gregata isolates from adzuki bean in Japan and from soybean in the United States. Plant Dis.67: 387–388.
Kobayashi, K., Yamamoto, H., Negishi, H. and Ogoshi, A. 1991. Formae speciales differentiation ofPhialophora gregata from adzuki bean and soybean in Japan. Ann. Phytopathol. Soc. Japan57: 225–231.
Michel, F. and Westhof, E. 1990. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J. Mol. Biol.216: 585–610.
Nishida, N., Blanz, P. A. and Sugiyama, J. 1993. The higher fungusProtomyces inouyei has two group I introns in the 18S rRNA gene. J. Mol. Evol.37: 25–28.
Sogin, M. L. and Edman, J. C. 1989. A self-splicing intron in the small subunit rRNA gene ofPneumocystis carinii. Nucleic Acids Res.17: 5349–5359.
White, T. J., Bruns, T., Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In. PCR protocols, A guide to methods and applications, (ed. by Innis, M. A., Gelfand, D. and White, T. J.), pp. 315–322. Academic Press, San Diego.
Yamada, T., Tamura, K., Aimi, T. and Songsri, P. 1994. Self-splicing group I introns in eukaryotic viruses. Nucleic Acid Res.22:2532–2537.
Yamamoto, H. 1995. Differential susceptibility ofPhialophora gregata ff. sp.adzukicola andsojae to antimicrobial chemicals. Mycoscience36: 125–126.
Yamamoto, H., Kobayashi, K. and Ogoshi, A. 1990. Isozyme polymorphism inPhialophora gregata isolates from adzuki bean and soybean in Japan. Ann. Phytopathol. Soc. Japan56: 584–590.
Yamamoto, H., Kobayashi, K. and Ogoshi, A. 1992. Genetic relatedness betweenPhialophora gregata f. sp.adzukicola andPhialophora gregata f. sp.sojae. Trans. Mycol. Soc. Japan33: 461–465.
Yamamoto, H. Kobayashi, K. and Ogoshi, A. 1993. Restriction fragment length polymorphisms in mitochondrial DNA ofPhialophora gregata in Japan. Trans. Mycol. Soc. Japan34: 465–471.
Yamamoto, H., Kobayashi, K. and Ogoshi, A. 1995. Characterization of the nuclear DNA ofPhialophora gregata ff. sp.adzukicola andsojae. Mycoscience36: 117–119.
Zuker, M. 1989. On finding all suboptimal foldings of an RNA molecule. Science244: 48–52.
Author information
Authors and Affiliations
About this article
Cite this article
Chen, W., Gray, L.E. & Grau, C.R. Characterization of a group I intron in the nuclera rDNA differentiatingPhialophora gregata f. sp.adzukicola fromP. gregata f. sp.sojae . Mycoscience 39, 279–283 (1998). https://doi.org/10.1007/BF02464009
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02464009