Abstract
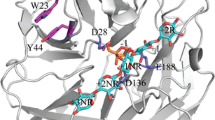
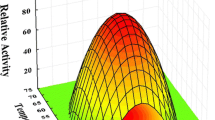
The genearfB encoding α-L-arabinofuranosidase B of the cellulolytic thermophileClostridium stercorarium was expressed inEscherichia coli from a 2.2-kbEcoRI DNA fragment. The recombinant gene product ArfB was purified by fast-performance liquid chromatography. It has a tetrameric structure with a monomeric relative molecular mass of 52 00. The optima for temperature and pH are 70°C and 5.0 respectively. The enzyme appears to have no metal cofactor requirement and is sensitive to sulfhydryl reagents. It hydrolyzes aryl and alkyl α-L-arabinofuranosides and cleaves arabinosyl side-chains from arabinoxylan (oat-spelt xylan) and from xylooligosaccharides produced by recombinant endoxylanase XynA from the same organism. The identity of the N-terminal amino acid sequences indicates that ArfB corresponds to the major α-arabinosidase activity present in the culture supernatant ofC. stecorarium.
Similar content being viewed by others
References
Bezalel L, Shoham Y, Rosenberg E (1993) Characterization and delignification activity of a thermostable α-L-arabinofuranosidase fromBacillus stearothermophilus. Appl Microbiol Biotechnol 40:57–62
Bronnenmeier K, Ebenbichler C, Staudenbauer WL (1990) Separation of the cellulolytic and xylanolytic enzymes ofClostridium stercorarium. J Chromatogr 521:301–310
Enzyme Nomenclature (1992) NC-IUBMB. Academic Press, New York
Grabski AC, Jeffries TW (1991) Production, purification, and characterization of β-(1-4)-endoxylanase ofStreptomyces roseiscleroticus. Appl Environ Microbiol 57:987–992
Greve LC, Labavitch JM, Hungate RE (1984) α-L-Arabinofuranosidase fromRuminococcus albus 8: purification and possible role in hydrolysis of Alfalfa cell wall. Appl Environ Microbiol 47:1135–1140
Hespell RB, O'Bryan PJ (1992) Purification and characterization of an α-L-arabinofuranosidase fromButyrivibrio fibrisolvens GS113. Appl Environ Microbiol 58:1082–1088
John MJ, Schmidt B, Schmidt J (1979) Purification and some properties of five endo-1,4-β-d-xylanases and a β-d-xylosidase produced by a strain ofAspergillus niger. Can J Biochem 57:125–134
Kaji A (1984)l-Arabinosidases. Adv Carbohydr Chem Biochem 42:383–394
Kormelink FJM, Searle-Van Leeuwen MJF, Wood TM, Voragen AGJ (1991) (1,4)-β-D-Arabinoxylan arabinofuranohydrolase: a novel enzyme in the bioconversion of arabinoxylan. Appl Microbiol Biotechnol 35:231–232
Madden RH (1984) Isolation and characterization ofClostridium stercorarium sp. nov., cellulolytic thermophile. Int J System Bacteriol 33:837–840
Matte A, Forsberg W (1992) Purification, characterization, and mode of action of endoxylanases 1 and 2 fromFibrobacter succinogenes S85. Appl Environ Microbiol 58:157–168
Poutanen K, Rättö M, Puls J, Viikari L (1987) Evaluation of different microbial xylanolytic systems. J Biotechnol 6:49–60
Schwarz WH, Bronnenmeier K, Gräbnitz F, Staudenbauer WL (1987) Activity staining of cellulases in polyacrylamide gels containing mixed linkage β-glucans. Anal Biochem 164:72–77
Schwarz WH, Schimming S, Staudenbauer WL (1988) Degradation of barley β-glucan by endoglucanase C ofClostridium thermocellum. Appl Microbiol Biotechnol 19:25–31
Schwarz WH, Adelsberger H, Jauris S, Hertel C, Funk B, Staudenbauer WL (1990) Xylan degradation by the thermophileClostridium stercorarium: cloning and expression of xylanase, β-d-xylosidase, and α-l-arabinofuranosidase genes inEscherichia coli. Biochem Biophys Res Commun 170:368–374
Shao W, Wiegel J (1992) Purification and characterization of a thermostable β-xylosidase fromThermoanaerobacter ethanolicus. J Bacteriol 174:5848–5853
Sedmark JJ, Grossberg SE (1977) A rapid, sensitive assay for protein using Coomassie brilliant blue G250. Anal Biochem 79:544–552
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160:87–112
Wood MW, McCrae SI (1986) Studies of two low-molecular-weight endo-(1 → 4)-β-d-xylanases constitutively synthesised by the cellulolytic fungusTrichoderma koningii. Carbohydr Res 148:321–330
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwarz, W.H., Bronnenmeier, K., Krause, B. et al. Debranching of arabinoxylan: properties of the thermoactive recombinant α-L-arabinofuranosidase fromClostridium stercorarium (ArfB). Appl Microbiol Biotechnol 43, 856–860 (1995). https://doi.org/10.1007/BF02431919
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02431919