Summary
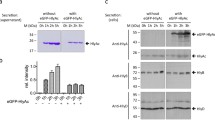
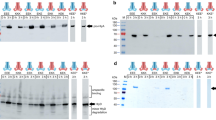
As a first step in the detailed analysis of the mechanism of secretion of haemolysin, we sought to identify sequences or domains within haemolysin A (HlyA) that are essential for its secretion. For this purpose we examined the properties of a deletion and Tn5 insertions into the region of theHlyA gene encoding the C-terminal part of the protein, since both of these are relatively simple to generate. We showed that removal of 27 amino acids from the C-terminus of HlyA is sufficient to inhibit secretion drastically, although the residual polypeptide is still haemolytically active. Cellular fractionation studies showed that haemolytic activity does not accumulate in large amounts within the periplasmic space during normal secretion. More significantly, activity does not appear to accumulate within this compartment when the export functionshlyB andhlyD are removed. These results are consistent with a mechanism in which interaction of the C-terminus of HlyA with the secretion machinery, located in the inner membrane, is followed by direct transfer of haemolysin to the medium.
Similar content being viewed by others
References
Bhakdi S, Mackman N, Nicaud J-M, Holland IB (1986)Escherichia coli haemolysin damages target cell membranes by generating transmembrane pores. Infect Immun 52:63–69
Chang A, Cohen S (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156
Felmlee T, Pellet S, Lee E-Y, Welch R (1985a)Escherichia coli haemolysin is released extracellularly without cleavage of a signal peptide J Bacteriol 163:88–93
Felmlee T, Pellet S, Welch R (1985b) Nucleotide sequence of anEscherichia coli chromosomal haemolysin. J Bacteriol 163:94–105
Garnier J, Osguthorpe D, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Hartlein M, Schiessl S, Wagner W, Rdest U, Kreft J, Goebel W (1983) Transport of haemolysin byEscherichia coli J Cell Biochem 22:87–97
Higgins C, Hiles I, Whalley K, Jamieson D (1985) Nucleotide binding by membrane components of bacterial periplasmic binding protein-dependent transport systems. EMBO J 4:1033–1040
Jackson M, Pratt J, Holland IB (1986) Intermediates in the assembly of the TonA polypeptide into the outer membrane ofEscherichia coli K12. J Mol Biol 189:477–486
Kyte J, Doolittle R (1982) A simple method for displaying the hydrophobic character of a protein. J Mol Biol 157:105–132
Larsen J, Gerdes K, Light J, Molin S (1984) Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene 28:45–54
Mackman N, Holland IB (1984a) Secretion of a 107 Kd polypeptide into the medium from a haemolyticEscherichia coli K12 strain. Mol Gen Genet 193:312–315
Mackman N, Holland IB (1984b) Functional characterization of a cloned haemolysin determinant fromEscherichia coli of human origin, encoding information for the secretion of a 107 Kd polypeptide. Mol Gen Genet 196:129–134
Mackman N, Nicaud J-M, Gray L, Holland IB (1985a) Genetical and functional organisation of theEscherichia coli determinant 2001. Mol Gen Genet 201:282–288
Mackman N, Nicaud J-M, Gray L, Holland IB (1985b) Identification of polypeptides required for the export of haemolysin 2001 fromEscherichia coli. Mol Gen Genet 201:529–536
Mackman N, Nicaud J-M, Gray L, Holland IB (1986) Secretion of haemolysin byEscherichia coli. Curr Top Microbiol Immunol 125:159–181
Nicaud J-M, Mackman N, Gray L, Holland IB (1985a) Regulation of haemolysin synthesis inEscherichia coli determined by Hly genes of human origin. Mol Gen Genet 199:111–116
Nicaud J-M, Mackman N, Gray L, Holland IB (1985b) Characterization of HlyC and mechanism of activation and secretion of haemolysin fromEscherichia coli 2001. FEBS Lett 187:339–344
Norrander J, Kempe T, Messing J (1983) Construction of improved M13 vectors using oligodeoxynucleotide directed mutagenesis. Gene 26:101–106
Nossal N, Heppel L (1966) The release of enzymes by osmotic shock fromEscherichia coli in exponential phase. J Biol Chem 241:3055–3062
O'Callaghan C, Morris A, Kirby S, Shingler A (1972) Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother 1:283–288
Peden K (1983) Revised sequence of the tetracycline-resistance gene of pBR322. Gene 22:277–280
Pugsley A, Schwartz M (1985) Export and secretion of proteins by bacteria, FEMS Microbiol Rev 32:3–38
Sanger F, Nicklen S, Coulson A (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Silhavy T, Benson S, Emr S (1983) Mechanisms of protein localization. Microbiol Rev 47:313–344
Stoker N, Pratt J, Spratt B (1983) Identification of therodA gene product ofEscherichia coli. J Bacteriol 155:854–859
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Yanisch-Perron, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Zabala J, Garcia-Lobo J, Diaz-Aroca E, de la Cruz F, Ortitz J (1984)Escherichia coli α-haemolysin synthesis and export genes are flanked by a direct repetition of IS91-like elements. Mol Gen Genet 197:90–97
Author information
Authors and Affiliations
Additional information
Communicated by J. Lengeler
Rights and permissions
About this article
Cite this article
Gray, L., Mackman, N., Nicaud, J.M. et al. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin fromEscherichia coli . Molec. Gen. Genet. 205, 127–133 (1986). https://doi.org/10.1007/BF02428042
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02428042