Abstract
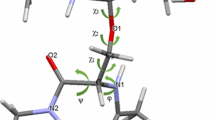
The oligomannose series of oligosaccharides from bovine thyroglobulin (BTG) and the variant surface glycoprotein (VSG) ofTrypanosoma brucei have been isolated and sequenced by1H NMR. The structure of Man9GlcNAc2, the parent molecule of the series, is shown below. Structural isomerism occurs within this series through the removal of residues D1, D2, D3, and C. Using spin-spin coupling and chemical shift data the rotamer distributions about the dihedral angle ω for the Manα1-6Man\ and Manα1-6Manα linkages were determined for each member of the series. It is shown that the dihedral angle ω of the Manα1-6Man\ linkage exhibits low flexibility with a preference for the ω = 180° conformation when residue D2 is present and high flexibility when this residue is absent. Flexibility of ω for the Manα1-6Manα is largely independent of primary sequence and is intermediate between the two Manoα1-6Man\ extremes, again with a preference for the ω = 180° conformation.

There are, however, data which indicate that removal of residue D3 may confer additional flexibility upon the dihedral angle ω of the Manα1-6Manα linkage. Molecular graphics modelling, together with chemical and enzymatic modification studies, suggest that the origin of the observed primary sequence dependence of the Manα1-6Man\ linkage arises from steric factors. On the basis of these observations taken together with previous work, it is postulated that recognition of individual oligomannose conformations may play a role in the control of N-linked oligosaccharide biosynthesis.
Similar content being viewed by others
Abbreviations
- AMBER:
-
assisted model building with energy refinement
- BTG:
-
bovine thyroglobulin
- COSY:
-
1H-1H correlation spectroscopy
- Endo-H:
-
endo-β-N-acetylglucosaminidase H
- NMR:
-
nuclear magnetic resonance
- NOE:
-
nuclear Overhauser effect
- NOESY:
-
two dimensional1H-1H nuclear Overhauser effect spectroscopy
- HOHAHA:
-
homonuclear Hartmann-Hahn spectroscopy
- HSEA:
-
hard sphere exo-anomeric effect, RECSY, multistep relayed correlation spectroscopy
- VSG:
-
variant surface glycoprotein
References
Altona C, Haasnoot CAG (1980) Prediction of anti and gauche vicinal proton-proton coupling constants in carbohydrates: a simple additivity rule for pyranose rings. Org Magn Res 13:417–429
Ashford D, Dwek RA, Welply JK, Amatayakul S, Homans SW, Lis H, Taylor GN, Sharon N, Rademacher TW (1987) The β1-2-D-Xylose and α1-3-L-Fucose substituted N-linked oligosaccharides fromErythrina cristagalli lectin. Isolation, characterization and comparison with other legume lectins. Eur J Biochem 166:311–320
Aue WP, Bartholdi E, Ernst RR (1976) Two-dimensional spectroscopy. Application to nuclear magnetic resonance. J Chem Phys 64:2229–2246
Berman E (1987) Conformational analysis and the fine structure of the cross peaks in phase-sensitive homonuclear two-dimensional correlated NMR spectra of oligosaccharides. Eur J Biochem 165:385–391
Berman E, Allerhand A (1981) Kinetics of α-D-mannopyranosyl linkages in hen ovalbumin glycopeptides as monitored by carbon-13 nuclear magnetic resonance spectroscopy. J Biol Chem 256:6657–6662
Biswas M, Sekharudu YC, Rao VS (1987) The conformation of the oligo-D-mannosidic type, and their interaction with glycans of concanavalin A: a computer-modelling study. Carbohydr Res 160:151–170
Bock K, Arnap J, Lonngren J (1982) The preferred conformation of oligosaccharides derived from the complex-type carbohydrate portions of glycoproteins. Eur J Biochem 129:171–178
Brisson JR, Carver JP (1983a) Solution conformation of alpha D(1-3)- and alpha D(1-6)-linked oligomannosides using proton nuclear magnetic resonance. Biochemistry 22:1362–1368
Brisson JR, Carver JP (1983b) Solution conformation of asparagine-linked oligosaccharides: alpha(1-2)-, alpha(1-3)-beta(1-2)- and beta(1-4)-linked units. Biochemistry 22:3671–3680
Brisson JR, Carver JP (1983c) Solution conformation of asparagine-linked oligosaccharides: alpha(1-6)-linked moiety. Biochemistry 22:3680–3686
Brisson JR, Carver JP (1983d) The relation of three-dimensional structure to biosynthesis in the N-linked oligosaccharides. Can J Biochem Cell Biol 61:1067–1078
Bush CA, Yan ZY, Rao BNN (1986) Conformational energy calculations and proton nuclear Overhauser enhancements reveal a unique conformation for blood group A oligosaccharides. J Am Chem Soc 108:6168–6173
Byrd JC, Tarentino AL, Maley F, Atkinson PH, Trimble RB (1982) Glycoprotein synthesis in yeast. Identification of Man8GlcNAc2 as an essential intermediate in oligosaccharide processing. J Biol Chem 257:14657–14666
Cohen RE, Ballou CE (1980) Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharide by proton nuclear magnetic resonance spectroscopy. Biochemistry 19:4345
Corio PL (1966) Structure of high resolution NMR spectra. Academic Press, New York, pp 299–305
Cumming DA, Carver JP (1987) Virtual and solution conformations of oligosaccharides. Biochemistry 26:6664–6676
Edge CJ, Singh UC, Bazzo R, Taylor GL, Dwek RA, Rademacher TW (1990) 500 Picosecond molecular dynamics in water of the Manα → 2Manα glycosidic linkage present in Asn-linked oligomannose type structures on glycoproteins. Biochemistry 29:1971–1974
Eich G, Bodenhausen G, Ernst RR (1982) Exploring nuclear spin systems by relayed magnetization transfer. J Am Chem Soc 104:3731–3732
Haasnoot CAG, DeLeeuw FAAM, Altona C (1980) The relation between proton-proton NMR coupling constants and substituent electronegativities. An empirical generalization of the Karplus equation Tetrahedron 36:2783–2792
Hassel O, Ottar B (1947) The structure of molecules containing cyclohexane or pyranose ring. Acta Chem Scand 1:929–943
Homans SW, Dwek RA, Fernandes DL, Rademacher TW (1983a) The use of two-dimensional correlated spectroscopy to obtain new assignments in the high-resolution1H nuclear magnetic resonance spectrum of the biantennary complex oligosaccharide isolated from human serum transferrin by hydrazinolysis. Biochim Biophys Acta 760:256–261
Homans SW, Dwek RA, Fernandes DL, Rademacher TW (1983b) Solution conformation of biantennary complex type oligosaccharides: determination of major conformers about the glycosidic linkages. FEBS Lett 164:231–235
Homans SW, Dwek RA, Fernandes DL, Rademacher TW (1984) Multiple-step relayed correlation spectroscopy: sequential resonance assignments in oligosaccharides. Proc Natl Acad Sci 81:6286–6289
Homans SW, Dwek RA, Boyd J, Mahmoudian M, Richards WG, Rademacher TW (1986) Conformational transitions in N-linked oligosaccharides. Biochemistry 25:6342–6350
Homans SW, Dwek RA, Boyd J, Soffe N, Rademacher TW (1987a) A method for the rapid assignment of1H NMR spectra of oligosaccharides using homonuclear Hartmann-Hahn spectroscopy. Proc Natl Acad Sci USA 84:1202–1205
Homans SW, Dwek RA, Rademacher TW (1987b) Tertiary structure in N-linked oligosaccharides. Biochemistry 26:6553–6560
Homans SW, Pastore A, Dwek RA, Rademacher TW (1987c) Structure and dynamics in oligomannose-type oligosaccharides. Biochemistry 26:6649–6655
Homans SW, Dwek RA, Rademacher TW (1987d) Solution conformations of N-linked oligosaccharides. Biochemistry 26:6571–6578
Homans SW, Edge CJ, Ferguson MAJ, Dwek RA, Rademacher TW (1989) Solution structure of the glycosyl-phosphatidylinositol membrane anchor glycan of trypanosoma brucei variant surface glycoprotein. Biochemistry 28:2881–2887
Jeener J, Meier BH, Bachmann P, Ernst RR (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys 71:4546–4553
Karplus M (1959) Contact Electron-Spin Coupling of Nuclear Magnetic Moments. J Chem Phys 30:11–15
Koerner TA, Prestegard JH, Yu RK (1987) Oligosaccharide structure by two-dimensional proton nuclear magnetic resonance spectroscopy. Methods Enzymol 138:38–59
Kornfeld R, Kornfeld S (1985) Assembly of asparagine-linked oligosaccharides. Ann Rev Biochem 54:631–664
Kumar A, Ernst RR, Wuethrich K (1980) A two-dimensional nuclear Overhauser exchange (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. BBRC 95:1–6
Lazzarino DA, Gabel CA (1989) Mannose processing is an important determinant in the assembly of phosphorylated high mannose-type oligosaccharides. J Biol Chem 264:5015–5023
Lemieux RU, Bock K, Delbaere LTJ, Koto S, Rao VS (1980) The conformations of oligosaccharides related to the ABH and Lewis human blood group determinants. Can J Chem 58:631–653
Lubas WA., Spiro RG (1988) Evaluation of the role of rat liver Golgiendo-alpha-D-mannosidase in processing N-linked oligosaccharides. J Biol Chem 263:3990–3998
Macura S, Huang Y, Suter D, Ernst RR (1981) Two-dimensional chemical exchange and cross-relaxation spectroscopy of coupled nuclear spins. J Mag Res 43:259–281
Maley F, Trimble RB (1981) Revision of the structure for anendo-β-N-acetylglucosaminidase H substrate using a novel modification of the Smith degradation. J Biol Chem 256:1088–1090
Marchessault RH, Perez S (1979) Conformation of the hydroxymethyl group in crystalline aldohexopyranoses. Biopolymers 18:2369–2374
Marion D, Wuthrich K (1983) Application of phase sensitive twodimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun 113:967–974
Nishida Y, Hori H, Ohrui H, Meguro H (1988) Proton NMR analysis for rotamerc distribution of the C(5)-C(6) bonds of D-glucopyranoses in solution. J Carb Chem 7:239–250
Ohruni H, Nishida Y, Watanabe M, Hori H, Meguro H (1985) Proton-NMR studies on (6R)- and (6S)-deuterated (1–6)-linked disaccharides: assignment of the preferred rotamers about C5-C6 bond of (1–6) disaccharides in solution. Tet Lett26:3251
Parekh RB, Tse AGC, Dwek RA, Williams AF, Rademacher TW (1987) Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J 6:1233–1244
Paulsen H, Peters T, Sinnwell V, Heume M, Meyer B (1986) Conformational analysis of the double pentasaccharide sequence of the “bisected” structure of N-glycoproteins. Carbohydr Res 156: 87–106
Rademacher TW Parekh RB, Dwek RA (1988) Glycobiology. Ann Rev Biochem 57:785–838
Schachter H (1986) Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Adv Exp Med Biol 205:53–85
Schachter H, Narasimhan S, Gleeson P, Vella G (1983) Glycosyltransferases involved in elongation of N-glycosidically linked oligosaccharides of the complex or N-acetyllactosamine type. Methods Enzymol 98:98–134
Van Halbeek H, Dorland L, Veldink GA, Vliegenthart JFG, Streker G, Michalski JC, Montreuil J, Hull WE (1980) A 500 MHz proton NMR study of urinary oligosaccharides from patients with mannosidosis. FEBS Lett 121:71–77
Vliegenthart JFG, Dorland L, Van Halbeek H (1983) High-resolution, H-nuclear magnetic resonance spectroscopy as a tool in the structural analysis of carbohydrates related to glycoproteins. Adv Carbohydr Chem Biochem 41:209–374
Wu GD, Serrianni AS, Barker R (1983) Stereoselective deuterium exchange of methylene protons in methyl tetrafuranosides: hydroxymethyl group conformations in methyl pentafuranosides. J Org Chem 48:1750–1757
Yan ZY, Rao BNN, Bush CA (1987) Influence of non-aqueous solvents on the conformation of blood group oligosaccharides. J Am Chem Soc 109:7663–7669
Zamze SE, Wooten WE, Ashford DA, Ferguson MAG, Dwek RA, Rademacher TW (1990) Characterisation of the asparagine-linked oligosaccharides fromTrypanosoma brucei type I variant surface glycoproteins. Eur J Biochem 187:657–663
Author information
Authors and Affiliations
Additional information
Offprint requests to: T W Rademacher
Rights and permissions
About this article
Cite this article
Wooten, E.W., Bazzo, R., Edge, C.J. et al. Primary sequence dependence of conformation in oligomannose oligosaccharides. Eur Biophys J 18, 139–148 (1990). https://doi.org/10.1007/BF02427373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02427373