Abstract
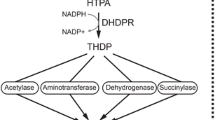
The first enzyme of the lysine-biosynthesis pathway, dihydrodipicolinate synthase (DHDPS; EC 4.2.1.52) has been purified and characterized inNicotiana sylvestris Speggazini et Comes. A purification scheme was developed for the native DHDPS that subsequently led to the purification to homogeneity of its subunits using two-dimensional gel electrophoresis. Subsequent elution of the purified polypeptide has opened the way for the production of rabbit polyclonal anti-DHDPS sera. The molecular weight of the enzyme was determined to be 164000 daltons (Da) by an electrophoretic method. By labeling with [14C]pyruvate, the enzyme was shown to be composed of four identical subunits of 38500 Da. Pyruvate acts as a stabilizing agent and contributes to the preservation of the tetrameric structure of the enzyme. The enzyme ofN. sylvestris is strongly inhibited by lysine with anI 0.5 of 15 μM; S-(2-aminoethyl)L-cysteine and γ-hydroxylysine, two lysine analogs, were found to be only weak inhibitors. An analog of pyruvate, 2-oxobutyrate, competitively inhibited the enzyme and was found to act at the level of the pyruvate-binding site. Dihydrodipicolinate synthase was localized in the chloroplast and identified as a soluble stromal enzyme by enzymatic and immunological methods. Its properties are compared with those known for other plant and bacterial DHDPS enzymes.
Similar content being viewed by others
Abbreviations
- ASS:
-
aspartic-β-semialdehyde
- Da:
-
daltons
- 2D:
-
two-dimensional gel electrophoresis
- DEAE:
-
cellulose-diethylaminoethylcellulose
- DHDPS:
-
dihydrodipicolinate synthase
- SDS:
-
sodium dodecyl sulfate
References
Bartlett, S.G., Grossman, A.R., Chua, N.N. (1982) In vitro synthesis and uptake of cytosplasmically-synthetized chloroplast proteins. In: Methods in chloroplast molecular biology, pp. 1081–1091, Edelmann ed. Elsevier, Amsterdam
Black, S., Wright, N.G. (1954a) Aspartic β-semialdehyde dehydrogenase and aspartic β-semialdehyde. J. Biol. Chem.213, 39–59
Black, S., Wright, N.G. (1954b) Homoserine dehydrogenase. J. Biol. Chem.213, 51–60
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254
Bryan, J.K. (1980) Synthesis of the aspartate family and branchedchain amino acids. In: The biochemistry of plants, a comprehensive treatise, vol. 5. Amino acids and deriatives, pp. 402–452, Miflin, B.J., ed. Academic Press, New York
Chamberlain, J.P. (1979) Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal. Biochem.98, 132–135
Cheshire, R.M., Miflin, B.J. (1975) The control of lysine biosynthesis in maize. Phytochemistry14, 695–698
Cleveland, D.W., Fischer, S.G., Kirschner, M.W., Laemmli, U.K. (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem.252, 1102–1106
Hallings, S.M., Stahly, D.P. (1976) Dihydrodipicolinic acid synthase ofBacillus licheniformis, quaternary structure, kinetics, and stability in the presence of sodium chloride and substrates. Biochim. Biophys. Acta452, 580–596
Harlow, E., Lane, D. (1988) Antibodies: a laboratory manual. Cold Spring Harbor, New York
Higgins, R.C., Dahmus, M.E. (1979) Rapid visualisation of protein bands in preparative SDS-polyacrylamide gels. Anal. Biochem.93, 267–260
Kumpaisal, R., Hashimoto, T., Yamada, Y. (1987) Purification and characterization of dihydrodipicolinate synthase from wheat suspension cultures. Plant Physiol.85, 145–151
Laemmli, U.K. (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature227, 680–685
LaRossa, R.A., Falco, S.C. (1984) Amino acid biosynthetic enzymes as target of herbicide action. Trends Biotechnol.2, 158–161
Mazelis, M., Miflin, B.J., Pratt, H.M. (1976) A chloroplast-localized diaminopimelate decarboxylase in higher plants. FEBS Lett.64, 197–200
Mazelis, M., Whatley, F.R., Whatley, J. (1977) The enzymology of lysine biosynthesis in higher plants; the occurrence, characterization and some regulatory properties of dihydrodipicolinate synthase. FEBS Lett.84, 236–240
Negrutiu, I., Cattoir-Reynaerts, A., Verbruggen, I., Jacobs, M. (1984) Lysine overproducer mutants with an altered dihydrodipicolinate synthase from protoplast culture ofNicotiana sylvestris (Spegazzini and Comes). Theor. Appl. Genet.68, 11–20
Oakley, B.R., Kirsch, D.R., Moris, N.R. (1980) A simplified ultrasensitive silver staining for detection of proteins in polyacrylamide gels. Anal. Biochem.105, 361–363
Richaud, F., Richaud, C., Ratet, P., Patte, J.C. (1986) Chromosomal location and nucleotide sequence of theEscherichia coli dap A gene. J. Bacteriol.166, 297–300
Shedlarsky, J.G., Gilvarg, C. (1970) The pyruvate-aspartic semialdehyde condensing enzyme ofEscherichia coli. J. Biol. Chem.245, 1362–1373
Thorum, W., Maurer, H.R. (1971) Molecular weight determination by polyacrylamide gel electrophoresis. In: Disc electrophoresis and related techniques of polyacrylamide gel electrophoresis, pp. 8–17, Maurer, H.R., ed. de Gruyter, Berlin
Wallsgrove, R.M., Mazelis, M. (1980) The enzymology of lysine biosynthesis in higher plants, complete localization of the regulatory enzyme dihydrodipicolinate synthase in the chloroplasts of spinach leaves. FEBS Lett.116, 189–192
Wallsgrove, R.M., Mazelis, M. (1981) Spinach leaf dihydrodipicolinate synthase: partial purification and characterization. Phytochemistry20, 2651–2655
Yamakura, F., Ikeda, Y., Kimura, K., Sasakawa, T. (1974) Partial purification and some properties of pyruvate-aspartic semialdehyde condensing enzyme from sporulatingBacillus subtilis. J. Biochem.76, 611–621
Yugari, Y., Gilvarg, C. (1965) The condensation step in diaminopimelate synthesis. J. Biol. Chem.240, 4710–4716
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghislain, M., Frankard, V. & Jacobs, M. Dihydrodipicolinate synthase ofnicotiana sylvestris, a chloroplast-localized enzyme of the lysine pathway. Planta 180, 480–486 (1990). https://doi.org/10.1007/BF02411444
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02411444