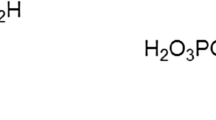Summary
Inhibition of seeded apatitic crystal growth by human salivary acidic proline-rich phosphoproteins (PRP) has been related to their adsorption onto the apatite seeds. The amino-terminal 30-residue segment of the PRP makes an important contribution to this adsorption. This peptide (PRP1(T1)) and its dephosphorylated analogue from PRP3 (PRP3(T1)DP) were prepared. They have identical sequences, except the phosphates at residues 8 and 22 in PRP1(T1) are absent from PRP3(T1)DP. Adsorption of these peptides onto hydroxyapatite and their effect on crystal growth from a defined supersaturated solution was studied. Adsorption behavior was adequately described by the Langmuir adsorption isotherm. The adsorption affinity constant of PRP1(T1) (K=20,200 ml/µmol) was more than 10 times the corresponding value for PRP3(T1)DP (1,800 ml/µmol), and similar to that of the parent protein, PRP1 (26,200 ml/µmol). Inhibition of crystal growth by the peptides was interpreted in terms of the fractional coverage of the maximum number of adsorption sites (as derived from the adsorption isotherms), suggesting that the molecules block, by adsorption, specific growth sites on these surfaces. Comparison of precipitation kinetics showed that PRP1(T1) is a more effective inhibitor than PRP3(T1)DP at the same initial concentration (10−6−10−7 M). However, on the basis of per mol adsorbed, PRP3(T1)DP displays a greater inhibitory activity; such a behavior is consistent with a more open molecular structure which blocks more growth sites per mol adsorbed than PRP1(T1). Because of its high affinity constant, preadsorbed PRP1(T1) remains in the condensed state in the supersaturated solution used, whereas the preadsorbed PRP3(T1)DP molecules desorb to some extent, resulting in a decrease in inhibitory activity. The results show that the amino-terminal segment of the PRP and the two phosphoserine residues present in this segment are particularly important in the function proposed for these proteins in the oral environment.
Similar content being viewed by others
References
Hay DI, Moreno E (1979) Macromolecular inhibitors of calcium phosphate precipitation in human saliva: their roles in providing a protective environment for the teeth. In: Kleinberg I, Ellison SA, and Mandel ID (eds) Proceedings “Saliva and Dental Caries.” Sp. Supp. Microbiology Abstracts, 45–58
Hay DI, Moreno EC, Schlesinger DH (1979) Phosphoprotein-inhibitors of calcium phosphate precipitation from salivary secretions. Inorg Persp Biol Med 2:271–285
Moreno EC, Varughese K, Hay DI (1979) Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int 28:7–16
Hay DI (1973) The interaction of human parotid salivary proteins with hydroxyapatite. Arch Oral Biol 18:1517–1529
Bennick A, Wong R, Cannon M (1977) Structure and biological activities of salivary acidic proline-rich phosphoproteins. In: Wasserman R et al (eds) Proc int symp on calciumbinding proteins and calcium function in health and disease. North-Holland, New York, 391–400
Moreno EC, Kresak M, Hay DI (1978) Adsorption of two human parotid salivary macromolecules on hydroxy-, fluorhydroxy- and fluorapatites. Archs Oral Biol 23:525–533
Kousvelari EE, Baratz RS, Burke B, Oppenheim FG (1980) Immunochemical identification and determination of proline-rich proteins in salivary secretions, enamel pellicle, and glandular tissue specimens. J Dent Res 59:1430–1438
Zahradnik RT, Moreno EC, Burke EJ (1976) Effect of salivary pellicle on enamel subsurface demineralization in vitro. J Dent Res 55:664–670
Schlesinger DH, Hay DI (1977) Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem 252:1689–1695
Schlesinger DH, Hay DI (1979) Peptides: structure and biological function. Gross E, Meienhofer J (eds) Proc sixth American peptide symp., Georgetown, Pierce Chemical Co, Rockford, Illinois, pp 133–136
Wong RSC, Hofmann T, Bennick A (1979) The complete primary structure of a proline-rich phosphoprotein from human saliva. J Biol Chem 254:4800–4808
Wong RSC, Bennick A (1980) The primary structure of a salivary calcium-binding proline-rich phosphoprotein (protein C), a possible precursor of a related salivary protein A. J Biol Chem 255:5943–5948
Bennick A, Cannon M, Madapallimattam G (1979) The nature of the hydroxyapatite-binding site in salivary acidic proline-rich proteins. Biochem J 183:115–126
Moreno EC, Kresak M, Hay DI (1982) Adsorption thermodynamics of acidic proline-rich human salivary proteins onto calcium apatites. J Biol Chem 257:2981–2989
Oppenheim FG, Hay DI, Franzblau C (1971) Proline-rich proteins from human parotid saliva. Biochemistry 10:4233–4238
Schlesinger DH, Hay DI (1981) Primary structure of the active tryptic fragments of human and monkey salivary anionic proline-rich proteins. Int J Pept Protein Res 17:34–41
Aoba T, Moreno EC (1984) Hydroxyapatite preparation and crystal growth on hydroxyapatite seeds. J Dent Res 63:874–880
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Moreno EC, Zahradnik RT, Glazman A, Hwu R (1977) Precipitation of hydroxyapatite from dilute solutions upon seeding. Calcif Tissue Res 24:47–57
Margolis HC, Varughese K, Moreno EC (1982) Effect of fluoride on crystal growth of calcium apatites in the presence of a salivary inhibitor. Calcif Tissue Int 34:S33-S40
Vogel AJ (1961) Quantitative inorganic analysis, 3rd ed. John Wiley and Sons, New York, p 810
Nancollas GH, Mohan MS (1970) The growth of hydroxyapatite crystals. Arch Oral Biol 15:731–745
Koutsoukos P, Amjad F, Tomson MB, Nancollas GH (1980) Crystallization of calcium phosphates. A constant composition study. J Am Chem Soc 102:1553–1557
Bennick A, McLaughlin AC, Grey AA, Madapallimattam G (1981) The location and nature of calcium-binding sites in salivary acidic proline-rich phosphoproteins. J Biol Chem 256:4741–4746
Hay DI, Schluckebier SK, Moreno EC (1982) Equilibrium dialysis and ultrafiltration studies of calcium and phosphate binding by human salivary proteins. Implications for salivary supersaturation with respect to calcium phosphate salts. Calcif Tissue Int 34:531–538
Hay DI, Moreno EC (1979) Differential adsorption and chemical affinities of proteins for apatite surfaces. J Dent Res (Special Issue B) 58:930–940
Meyer JL, Nancollas GH (1973) The influence of multidentate organic phosphonates on the crystal growth of hydroxyapatite. Calcif Tissue Res 13:295–303
Kresak M, Moreno EC, Zahradnik RT, Hay DI (1977) Adsorption of amino acids onto hydroxyapatite. J Coll Int Sci 59:283–292
Jung A, Bisaz S, Fleisch H (1973) The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tissue Res 11:269–280
Meyer JL, McCall JT, Smith LH (1974) Inhibition of calcium phosphate crystallization by nucleoside phosphates. Calcif Tissue Res 15:287–293
Moreno EC, Kresak M, Hay DI (1984) Adsorption of molecules of biological interest onto hydroxyapatite. Calcif Tissue Int 36:48–59
Reynolds EC, Riley PF, Storey E (1982) Phosphoprotein inhibition of hydroxyapatite dissolution. Calcif Tissue Int 34:S52-S56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aoba, T., Moreno, E.C. & Hay, D.I. Inhibition of apatite crystal growth by the amino-terminal segment of human salivary acidic proline-rich proteins. Calcif Tissue Int 36, 651–658 (1984). https://doi.org/10.1007/BF02405385
Issue Date:
DOI: https://doi.org/10.1007/BF02405385