Abstract
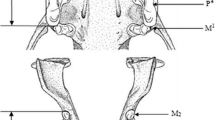
In a sample of 24 skulls of the rare colobine monkey of China,Rhinopithecus roxellana, agenesis of the permanent upper third premolar was observed in 8 specimens (33%). Two of the individuals (8% of the sample) showed unilateral agenesis, while six (25%) showed bilateral agenesis. All of the affected individuals appear to have originated from two areas in central Sichuan Province in China, Moupin (Baoxing) and Wen Chuan, that lie approximately 120 km apart on the western rim of the Sichuan Basin. In this species agenesis of the upper third premolar was generally accompanied by rotation of the upper fourth premolar, but not by any other observable variation in tooth size, morphology, or number. Further, dental agenesis in this species appears to have had no effect craniofacial morphology. In certain human populations, a high prevalence of dental agenesis has been associated with small population effects and genetic isolation. Premolar agenesis inR. roxellana can probably be traced to a similar origin. Reconstruction of the evolutionary history of the species indicates that a severe reduction of the geographic range of the species occurred as a result of climatic deterioration during the Late Pleistocene. Although large numbers of populations appear to have become extinct, others survived to give rise to the modern populations of the species that inhabit China today. The two populations showing a high prevalence of premolar agenesis appear to have originated from one that passed through a population bottleneck and suffered the consequences of founder effect in the Late Pleistocene. This interpretation is supported by evidence of dental agenesis in a population of an insectivore species from the same region and by the fact that premolar agenesis is not found in any of the other species ofRhinopithecus. There is no evidence to support the interpretation that dental agenesis inR. roxellana is due to natural selection, mutation or an evolutionary force other than a small population effect.
Similar content being viewed by others
References
Brace, C. L., K. R. Rosenberg, &K. D. Hunt, 1987. Gradual change in human tooth size in the Late Pleistocene and Post-Pleistocene.Evolution, 41: 705–720.
Colbert, E. H. &D. A. Hooijer, 1953. Pleistocene mammals from the limestone fissures of Szechuan, China.Bull. Amer. Mus. Nat. Hist., 102: 1–144.
Colyer, F., 1936.Variations and Diseases of the Teeth of Animals. John Bale, Sons & Danielsson, London.
Graber, L. W., 1978. Congenital absence of teeth: a review with emphasis on inheritance patterns.J. Amer. Dent. Ass., 96: 266–275.
Grahnén, H., 1956. Hypodontia in the permanent dentition.Odont. Rev., 7 (Suppl. 3): 1–100.
Hooijer, D. A., 1952. Notes on the dentition of the golden monkey,Rhinopithecus.J. Mammal., 33: 258–260.
Hylander, W. L. &R. F. Kay, 1975. Maxillary premolar reduction in the golden monkey (Rhinopithecus roxellanae).J. Dent. Res., 54: 1242.
Jablonski, N. G., 1991. Dental variations in species ofRhinopithecus and their relationship to the evolution of the genus.Amer. J. Phys. Anthropol., (Suppl. 12): 98. (Abstract)
———— &Y.-M. Gu, 1991. A reassessment ofMegamacaca lantianensis, a large monkey from the Pleistocene of north-central China.J. Human Evol., 20: 51–66.
———— &Y.-R. Pan, 1988. The evolution and palaeobiogeography of monkeys in China. In:The Palaeoenvironment of East Asia from the Mid-Tertiary, Vol. II,P. Whyte,J. Aigner,N. G. Jablonski,G. Taylor,D. Walker,P.-X. Wang, andC. L. So (eds.), Centre of Asian Studies, Hong Kong, pp. 849–867.
Johr, A. C., 1934. Reduktionserscheinungen an den oberen seitlichen Schneiderzflhnen.Arch. J. Klaus-Stiftung, 9: 6–131.
Kirveskari, P., H. Hansson, B. Hedegard, &V. Karlsson, 1978. Crown size and hypodontia in the permanent dentition of modern Skolt Lapps.Amer. J. Phys. Anthropol., 48: 107–112.
Lavelle, C. L. B. &W. J. Moore, 1973. The incidence of agenesis and polygenesis in the primate dentition.Amer. J. Phys. Anthropol., 38: 671–680.
Mahaney, M. C., T. M. Fujiwara, &K. Morgan, 1990. Dental agenesis in the Dariusleut Hutterite Brethren: Comparisons to selected caucasoid population surveys.Amer. J. Phys. Anthropol., 82: 165–177.
Osgood, W. H., 1937. Variable dentition in a Chinese insectivore.Field Mus. Nat. Hist., Zool., 20: 365–368.
Suarez, B. K. &M. A. Spence, 1974. The genetics of hypodontia.J. Dent. Res., 53: 781–785.
Thomsen, S. O., 1953. Missing teeth with special reference to the population of Tristan da Cunha.Amer. J. Phys. Anthropol., 10: 155–167.
Author information
Authors and Affiliations
About this article
Cite this article
Jablonski, N.G. Dental agenesis as evidence of possible genetic isolation in the colobine monkey,Rhinopithecus roxellana . Primates 33, 371–376 (1992). https://doi.org/10.1007/BF02381198
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02381198