Abstract
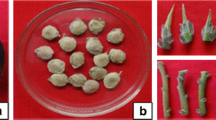
Rapid clonal propagation of Bolaina blanca (Guazuma crinita Mart.) was established by the subculturing of the shoot-tips from aseptically germinated seedlings on woody plant medium (WPM) supplemented with trans-zeatin [trans-6-(4-Hydroxy-3-methylbut-2-enylamino)purine] (ZEA). After 45 days of culture, a seven-fold multiplication rate was achieved on WPM containing 10 μM of ZEA. Obtained shoots were simultaneously elongated and rooted on WPM containing 1 μM of kinetin [6-furfurylaminopurine] (KIN). After 60 days of culturing the growth of shoots was evident, and high rooting percentages were obtained. The plantlets were transferred into pots with vermiculite and acclimatized successfully in plastic boxes with transparent cover inside the growth cabinet.
Similar content being viewed by others
Literature cited
Banko, T.J. and Stefani, M. (1989)In vitro propagation ofOxydendrum arboreum from mature trees. Hortic. Sci. 24: 683–685.
Bonga, J.M. and Aderkas, P. von (1992)In vitro Culture of Trees. 236 pp, Kluwer Academic Publishers, Dordrecht.
Chalupa, V. (1984)In vitro propagation of oak (Quercus robur L.) and linden (Tilia cordata Mill.). Biol. Plant. 26: 374–377.
Coleman, G.D. and Ernst, S.G. (1989)In vitro shoot regeneration ofPopulus deltoides: effect of cytokinin and genotype. Plant Cell Rep. 8: 459–462.
Encarnación, F. (1983) Nomenclature de las Especies Forestales Comunes en el Perú. 149 pp, Proyecto PNUD/FAO/PER/81/002 Fortalecimiento de los Programas de Desarrollo Forestal en la Selva Central, Documento de Trabajo No. 7, Lima-Perú.
Evers, P.W., Donkers, J., Prat, A., and Vermeer, E. (1988) Micropropagation of forest trees through tissue culture. 84 pp, Centre for Agricultural Publishing and Documentation (Pudoc), Wageningen.
Fossard, R.A. De, Bennett, M.T., Gorst, J.R., and Bourne, R.A. (1978) Tissue culture propagation ofEucalyptus ficifolia F. Muell. Proc. Int. Plant Prop. Soc. 28: 427–435.
Freytag, G.F. (1951) A revision of the genusGuazuma. Ceiba (Hond.) 1: 193–225.
Gamborg, O.L., Miller, R.A., and Ojima, K. (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158.
Gupta, P.K., Nadgir, A.L., Mascarenhas, A.F., and Jaganathan, V. (1980) Tissue culture of forest trees: Clonal multiplication ofTectona grandis L. (teak) by tissue culture. Plant Sci. Lett. 17: 259–268.
Ishii, K. and Maruyama, E. (1992) Tissue culture of some tropical tree species in Peru.In Plant Tissue Culture and Gene Manipulation for Breeding and Formation of Phytochemicals. Oono, K., Hirabayashi, T., Kikuchi, S., Handa, H., and Hajiwara, K. (eds.), 395 pp, National Institute of Agrobiological Resources, Tsukuba, 219–223.
Ishii, K. and Maruyama, E. (1994) Micropropagation of tropical forest trees.In International Wood Biotechnology Symposium. 123 pp, Tokyo, 85–90.
Jain, A.K., Dandin, S.B., and Sengupta, K. (1990)In vitro propagation through axillary bud multiplication in different mulberry genotypes. Plant Cell Rep. 8: 737–740.
Lloyd, G. and McCown, B. (1980) Commercially-feasible micropropagation of mountain laurel,Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Intern. Plant Prop. Soc. 30: 421–427.
Maruyama, E., Yokoyama, T., and Migita, K. (1989a) Effect of temperature and pre-heating on germination ofGuazuma crinita Mart. andGuazuma ulmifolia Lam. seeds. J. Jpn For. Soc. 71: 65–68.
Maruyama, E., Ishii, K., Saito, A., and Migita, K. (1989b) Screening of suitable sterilization of explants and proper media for tissue culture of eleven tree species of Perú-Amazon Forest. J. Agric. Sci. The Tokyo Univ. of Agric. 33: 252–261.
Maruyama, E., Ishii, K., Saito, A., and Migita, K. (1989c)In vitro multiple shoot formation and callus culture from shoot-tip of aseptically germinated seedlings of tornillo (Cedrelinga catenaeformis Ducke). Agric. Sci. The Tokyo Univ. of Agric. 33: 252–261.
Maruyama, E., Ishii, K., Saito, A., and Migita, K. (1989d) Micropropagation of cedro (Cedrela odorata L.) by shoot-tip culture. J. Jpn. For. Soc. 71: 329–331.
Maruyama, E., Ishii, K., Saito, A., and Ohba, K. (1993) Micropropagation of jacaranda (Jacaranda mimosaefolia D. Don) by shoot-tip culture. J. Jpn. For. Soc. 75: 346–349.
Minocha, S.C. (1987) Plant growth regulators and morphogenesis in cell and tissue culture of forest trees.In Cell and Tissue Culture in Forestry, Vol. 1, General Principles and Biotechnology. Bonga, J.M. and Durzan, D.J. (eds.), 422 pp, Martinus Nijhoff Publishers, Dordrecht, 50–66.
Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473–497.
Nehra, N.S. and Kartha, K.K. (1994) Meristem and shoot tip culture: requirements and applications.In Plant Cell and Tissue Culture. Vasil, I.K. and Thorpe, T.A. (eds.), 593 pp, Kluwer Academic Publishers, Dordrecht, 37–70.
Paranjothy, K. (1987)Hevea tissue culture.In Cell and Tissue Culture in Forestry, Vol. 3, Case Histories: Gymnosperms, Angiosperms and Palms. Bonga, J.M. and Durzan, D.J. (eds.), 416 pp, Martinus Nijhoff Publishers, Dordrecht, 326–337.
Šebánek, J., Sladký, Z., Procházka, S., Kutáček, M., Luxová, M., and Erdelská, O. (1991) Experimental Morphogenesis and Integration of Plants, Developments in Crop Sciences 18, 418 pp, Elsevier Science Publishers, Amsterdam.
Wareing, P.F. and Phillips, I.D.J. (1970) The Control of Growth & Differentiation in Plants. 303 pp, Pergamon Press, Oxford.
Zaerr, J.B. and Mapes, M.O. (1982) Action of growth regulators.In Tissue Culture in Forestry. Bonga, J.M. and Durzan, D.J. (eds.), 420 pp, Martinus Nijhoff/Dr W. Junk Publishers, The Hague, 231–255.
Author information
Authors and Affiliations
About this article
Cite this article
Maruyama, E., Ishii, K., Kinoshita, I. et al. Micropropagation of Bolaina blanca (Guazuma crinita Mart.), a fast-growing tree in the Amazon region. J. For. Res. 1, 211–217 (1996). https://doi.org/10.1007/BF02348327
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02348327