Abstract
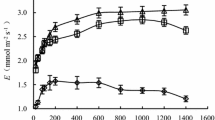
The adaptation of Camellia rusticana, an evergreen broad-leaved shrub found in areas of heavy snowfall in Japan, to heavy snowfall environments, and the mechanisms by which it is damaged in winter above the snow, were investigated. The stomatal response and photosynthetic characteristics of C. rusticana were compared to those of Camellia japonica found in areas of light snowfall. In field conditions, the mean net photosynthesis of C. rusticana at photon flux density (PFD) over 200 μmol m−2s−1 (Pn(>200). was 50% larger than that of C. japonica, but in both light saturated and CO2 saturated conditions, the O2 evolution rate (Pc) of C. rusticana was not different from that of C. japonica. Mean leaf conductance at PFD over 200 μmol m−2s−1 (gl(>200)) was about 100% larger than that of C. japonica in the field. The Pn(>200)) was 50% ratio of C. rusticana was 37% higher than that of C. japonica which suggests that C. rusticana's larger Pn(>200) can be explained by its larger gl(>200). When C. rusticana trees wintering underneath the snow were projected above it, the leaves of these plants showed serious drought within five days in non-freezing conditions. Their Pc and the maximum stomatal conductance decreased by half and did not recover. The leaves of C. rusticana showed larger gl(>200) and a less sensitive stomatal response to the decrease of leaf water potential than that of C. japonica. The stomata characteristics of C. rusticana caused larger net photosynthesis than that of C. japonica during the no snow period, and caused the need for snow cover in winter as protector from winter drought.
Similar content being viewed by others
References
Baig M. N. & Tranquillini W. (1976) Studies on upper timberline: morphology and anatomy of Norway spruce (Picea abies) and stone pine (Pinus cembra) needles from various habitat conditions. Canadian Journal of Botany 54: 1622–1632.
Bliss L. C. (1962) Adaptations of arctic and alpine plants to environmental conditions. Arctic 15: 117–144.
Cowan I. R. (1977) Stomatal behaviour and environment. Advances in Botanical Research 4: 117–228.
Cowan I. R. (1982) Regulation of water use in relation to carbon gain in higher plants. In: Encyclopedia of Plant Physiology, vol. 12A (eds O. L. Lange, P. S. Nobel, C. B. Osmond & H. Ziegler) pp. 589–613. Springer-Verlag. Berlin.
Elfving D. C., Kaufmann M. R. & Hall A. E. (1972) Interpreting leaf water potential measurements with a model of a soil-plant-atmosphere continuum. Physiologia Plantarum 27: 161–168.
Fukuda A., Yamatani S., Kobata T. & Imaki T. (1993a) Decrease of leaf water potential and leaf injury of tea plant (Thea sinensis L.) subject to cold winter wind in Sanin region of Japan. I. Response of leaf water potential and leaf injury under field conditions. Japanese Journal of Crop Science 62: 188–192.
Fukuda A., Yamatani S., Kobata T. & Imaki T. (1993b) Decrease of leaf water potential and leaf injury of tea plant (Thea sinensis L.) subject to cold winter wind in Sanin region of Japan. II. Increase of water flow resistance inside the plant contributes to decrease of leaf water potential. Japanese Journal of Crop Science 62: 193–198.
Hagiya K. & Ishizawa S. (1968) On some physiological aspects of the bush and snow camellia. In: Camellias of Japan (ed. T. Tuyama) pp. 27–32. Takeda Science Foundation. Osaka.
Horikawa Y. (1972) Atlas of the Japanese Flora I. Gakken, Tokyo.
Ishizawa S. (1978) Climatic factors affecting the distribution of Snow-Camellia (Camellia rusticana Honda). In: Plant Ecology to the Memory of Dr. Kuniji Yoshioka pp. 298–306. Society of Tohoku Plant Ecology, Sendai (in Japanese).
Japan Meteorological Agency (1993) Climatic Atlas of Japan 1990. The Japan Mereorological Agency, Tokyo.
Jones H. G. (1976) Crop characteristics and the ratio between assimilation and transpiration. Journal of applied Ecology 13: 605–622.
Kume A. & Ino Y. (1993) Comparison of ecophysiological responses to heavy snow in two varieties of Aucuba japonica with different distributing areas. Ecological Research 8: 111–121.
Kramer P. J. (1942) Species differences with respect to water absorption at low soil temperatures. American Journal of Botany 29: 828–832.
Larcher W. (1963) Zür spätwinterlichen Erschwerung der Wasserbilanz von Holzpflanzen an der Waldgrenze. Berichte des Naturwissenschaftlich-Medizinischen Vereins in Innsbruck. 53: 125–137.
Oshima Y. (1961) Ecological studies of Sasa communities. IV. Dry matter production and distribution of products among various organs in Sasa kurilensis community. Botanical Magazine Tokyo 74: 473–479.
Pearcy R. W. (1988) Photosynthetic utilization of lightflecks by understorey plants. Australian Journal of Plant Physiology 15: 223–238.
Sakai A. (1978) Freezing tolerance of evergreen and deciduous broadleaved trees in Japan with reference to tree regions. Low Temperature Science Series B 36: 1–19.
Sakai A. (1982) Freezing resistance of ornamental trees and shrubs. Journal of American Society of Horticultural Science 107: 572–581.
Sakai A. & Hakoda N. (1979) Cold hardiness of the genusCamellia. Journal of American Society of Horticultural Science 104: 53–57.
Sakai A. & Larcher W. (1987) Frost Survival of Plants. In: Ecological Studies Vol. 62. Springer-Verlag, Berlin.
Slatyer R. O. (1967) Plant Water Relationship. Academic Press, New York.
Shimada H. & Hisada Y. (1966) Anatomical studies on the leaves of Camellia japonica andC. rusticana. The Journal of Japanese Botany 41: 33–36 (in Japanese with English summary).
Tranquillini W. (1982) Frost-drought and its ecological significance. In: Encyclopedia of Plant Physiology, vol. 12B (eds O. L. Lange, P. S. Nobel, C. B. Osmond & H. Ziegler) pp. 379–400. Springer-Verlag, Berlin.
Tuyama T. (1956) On some morphological features of Camellia japonica andC. rusticana. The Journal of Japanese Botany 31: 225–228 (in Japanese).
Walker D. A. (1987) The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis. Oxygraphics, Sheffield.
Waring R. H. & Cleary B. D. (1967) Plant moisture stress: evaluation by pressure bomb. Science 155: 1248–1254.
Author information
Authors and Affiliations
About this article
Cite this article
Kume, A., Tanaka, C. Adaptation of stomatal response of Camellia rusticana to a heavy snowfall environment: Winter drought and net photosynthesis. Ecol. Res. 11, 207–216 (1996). https://doi.org/10.1007/BF02347687
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02347687