Abstract
Objective and Design: A novel immunomodulating drug, leflunomide has been shown recently to be effective and well tolerated in patients suffering from rheumatoid arthritis (RA). The present study evaluated the effect of the drug on cell adhesion in RA.
Material and Treatment: Peripheral blood and synovial fluid mononuclear cells were obtained from a clinical trial, undertaken primarily to evaluate the efficacy and pharmacokinetic profile of multiple-dose pulsing leflunomide therapy in RA patients. PB MNC and corresponding synovial fluid (SF) MNC for in vitro homotypic aggregation (HA) assay were obtained from healthy volunteers and RA patients with active disease not treated with leflunomide in vivo.
Methods: Expression of activation antigens (CD25, CD54, CD69, CD71, HLA-DR) on peripheral blood mononuclear cells (PB MNC), as well as ex vivo ability of cells to aggregate spontaneously were determined in patients before entering into the clinical trial and at the end of 6 months treatment. HA was measured by aggregation in vitro. Data were compared by Student's t-test.
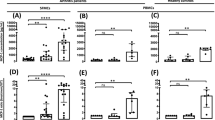
Results: There was a decreased expression of activation antigens and decreased spontaneous MNC clustering after leflunomide therapy. We found in the in vitro study that HA of PB and SF MNC was mainly mediated through β2-integrin molecules. The active metabolite of leflunomide, A77 1726, effectively suppressed both spontaneous and phorbol-ester (PMA)-induced HA. Disruption of cell aggregates by A77 1726 was dose-dependent and, most likely, unrelated to the quantitative modulation of integrin receptors.
Conclusions: Results from this study support the idea that leflunomide elicits its immunomodulatory action, at least partially, by modulating the adhesion process.
Similar content being viewed by others
References
Bartlett RR, Schleyerbach R. Immunopharmacological profile of a novel isoxazol derivate, HWA 486, with potential antirheumatic activity. I. Disease modifying action on adjuvant arthritis of the rat. Int J Immunopharm 1985;7:7–18.
Bartlett RR, Dimitrijevic M, Mattar T, Zielinski T, Germann T, Rude E, et al. Leflunomide (HWA 486), a novel immunomodulating compound for the treatment of auto-immune disorders and reactions leading to transplantation rejection. Agents Actions 1991;32:10–21.
Ogawa T, Inazu M, Gotoh K, Hayashi S. Effects of leflunomide on glomerulonephritis induced by antibasement membrane antibody in rats. Agents Actions 1990;31:321–8.
Smith-Long L, Glaser B, Weimer LK, Miller ST, Robertson SM, Aoki KR, et al. Efficacy of novel immunomodulators leflunomide and rapamycin in autoimmune uveitis. FASEB J 1992;6:A1048.
Xiao F, Chong AS-F, Bartlett RR, Williams JW, Leflunomide: a promising immunosuppressant in transplantation. In: Thompson AW, Starzl TE, editors. Immunosuppressive Drugs. Developments in anti-rejection therapy. London: Edward Arnold, 1994;203–12.
Mladenovic V, Domljan Z, Rozman B, Jajic I, Mihajlovic, D, Dordevic J, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomised, placebo-controlled, phase II study. Arthr Rheum 1995;38:1595–603.
Bartlett RR, Anagnostopulos H, Zielinski T, Mattar T, Schleyerbach R. Effects of leflunomide on immune responses and models of inflammation. Springer Sem Immunopathol 1993;14:381–94.
Bartlett RR, Zielinski T, Campion G, Musikic P, Schleyerbach R, Schorlemmer H-U. Chapter 20: Leflunomide: A novel immunomodulatory drug. In: Lewis AJ, Furst D, editors. Non-Steroidal Anti-Inflammatory Drugs II. New York: Marcel Dekker, 1994:349–66.
Zielinski T, Muller HJ, Schleyerbach R, Bartlett RR. Differential effects of leflunomide on leukocytes: inhibition of rat in vivo adhesion and in vitro oxidative burst without affecting surface marker modulation. Agents Actions 1994;41:C276–8.
Mattar T, Kochhar K, Bartlett RR, Bremer EG, Finnegan A. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett 1993;334:161–4.
Nikcevich DA, Finnegan A, Chong ASF, Williams JW, Bremer EG. Inhibition of interleukin 2 (IL-2)-stimulated tyrosine kinase activity by leflunomide. Agents Actions 1994;41:C279–82.
Zielinski T, Zeitter D, Mullner S, Bartlett RR. Leflunomide, a reversible inhibitor of pyrimidine biosynthesis? Inflamm Res 1995;44 Suppl 2:S207–8.
Williamson RA, Yea CM, Robson PA, Curnock AP, Gadher S, Hambleton AB, et al. Dihydroorotate dehydrogenase is a high affinity binding protein for A77 1726 and mediator of a range of biological effects of the immunomodulatory compound. J Biol Chem 1995;270:22467–72.
Pitzalis C, Kingsley GH, Covelli M, Meliconi R, Markey A, Panayi GS. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol 1991;21:369–76.
Haynes BF, Hale LP, Patton KL, Martin ME, McCallum RM. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Arthr Rheum 1991;34:1434–43.
Springer TA. Adhesion receptors of the immune system. Nature 1990;346:425–33.
Carlos TM, Harlan JM, Leukocyte-endothelial adhesion molecules. Blood 1994;84:2068–101.
Oppenheimer-Marks N, Davis LS, Brogue DT, Ramberg J, Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol 1991;147:2913–21.
Oppenheimer-Marks N, Lipsky PE. The role of cell adhesion in the evolution of inflammatory arthritis. In: Wegner CD, editor. Adhesive Molecules. London: Academic Press, 1994: 141–61.
Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell 1992;69:11–25.
van Seventer GA, Newman W, Shimizu Y, Nutman TB, Tanaka Y, Horgan KJ, et al. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: co-stimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med 1991;174:901–13.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthr Rheum 1988;31:315–24.
Mueller J, Brown del Re G, Buerki H, Keller HU, Hess MW, Cotier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol 1975;5:270–4.
Rothlein R, Springer TA. The requirement for leukocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med 1986;163:1132–49.
Potocnik AJ, Kinne R, Menninger H, Zacher J, Emmrich F, Kroczek RA. Expression of activation antigens on T cells in rheumatoid arthritis patients. Scand J Immunol 1990;31:213–24.
Hovendes J, Gaudernack G, Kvien TK, Egeland T. Expression of activation markers on CD4+ and CD8+ cells from synovial fluid, synovial tissue and peripheral blood of patients with inflammatory arthritides. Scand J Immunol 1989;29: 631–9.
Patarroyo M. Leukocyte adhesion in host defence and tissue injury. Clin Immunol Immunopathol 1991;60:333–48.
Sanders ME, Makgoba WM, Sharrow SO, Stephany D, Springer TA, Young HA, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2 and LFA-1) and three other molecules (UCHL 1, CDw29 and Ppg-1) and have enhanced IFN-γ production. J Immunol 1988;140:1401–7.
Hemler ME, Glass D, Coblun JS, Jacobson JG. Very late activation antigens on rheumatoid synovial fluid T cells: association with stages of T cell activation. J Clin Invest 1986;78:696–702.
Locey BJ, Rogers CM, Todd RF. The role of CD11/CD18 integrin molecules in neutrophil and monocyte homotypic adhesion. In: Knapp W, Dorken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, von dem Borne AEGK, editors. Leukocyte Typing IV. White Cell Differentiation Antigens. Oxford: Oxford University Press, 1989:555–8.
Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Ann Rev Immunol 1991;9:27–66.
Pardi R, Inyerardi L, Bender J. Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travellers. Immunol Today 1992;13:224–30.
Hogg N, Berlin C. Structures and function of adhesion receptors in leukocyte trafficking. Immunol Today 1995;16: 327–30.
Pavlovic MD, Colic M, Pejnovic N, Tamatani T, Miyasaka M, Dujic A. A novel anti-rat CD18 monoclonal antibody triggers lymphocyte homotypic aggregation and granulocyte adhesion to plastic: different intracellular signaling pathways in resting versus activated thymocytes. Eur J Immunol 1994;24: 1640–8.
Patarroyo M, Makgoba MW. Leukocyte adhesion to cells: molecular basis, physiological relevance and abnormalities. Scand J Immunol 1989;30:129–64.
Hibbs ML, Xu H, Stocker SA, Springer TA. Regulation of adhesion to ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science 1991;251:1537–42.
Burn P, Kupfer A, Singer SJ. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci USA 1988;85:497–501.
Koopman G, Kooyk Y, de Graaf M, Meijer CJLM, Figdor CG, Pals ST. Triggering of CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol 1990;145:3589–93.
Pals ST. CD44, a family of molecules involved in lymphocyte activation and inflammation. Rheumatol in Europe 1995;24: 35–7.
Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today 1995;16:479–83.
Author information
Authors and Affiliations
Additional information
accepted by M. J. Parnham
Rights and permissions
About this article
Cite this article
Dimitrijevic, M., Bartlett, R.R. Leflunomide, a novel immunomodulating drug, inhibits homotypic adhesion of peripheral blood and synovial fluid mononuclear cells in rheumatoid arthritis. Inflamm Res 45, 550–556 (1996). https://doi.org/10.1007/BF02342226
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02342226