Abstract
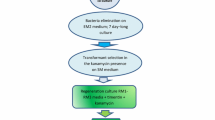
A method for genetic transformation of lisianthus by co-cultivation ofin vitro grown leaves withAgrobacterium tumefaciens is described. Two strains, A281 and EHA105, both carrying the plasmid pKIWI105 capable of expressing the GUS gene were used in preliminary tests. A281 had higher transformation efficiency in terms of transient and stable expression. The efficiency of the strain EHA105 could be improved by including 2iP in co-cultivation and selection media, but never reached that of the oncogenic strain A281. An expression cassette containing the nucleoprotein gene of TSWV, carried by a binary plasmid, was introduced into EHA105 and used to transform lisianthus. After transformation and regeneration, the potentially useful transgene was present in seven plants derived from independent events of transformation and the presence of the transgenic protein was detected in one of them.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- Cm:
-
chloramphenicol
- Cx:
-
cefotaxime
- 2,4-d :
-
2,4-dichlorophenoxy acetic acid
- GUS:
-
β-glucuronidase
- IAA:
-
indole-3-acetic acid
- 2iP:
-
2-isopentenyl adenine
- Km:
-
kanamycin
- NAA:
-
2-napthalene acetic acid
- NPTII:
-
neomycin phosphotransferase II
- PCR:
-
polymerase chain reaction
- Rif:
-
rifampicin
References
Bevan M (1984) BinaryAgrobacterium vectors for plant transformation. Nucleic Acids Res. 12: 8711–8721
Dong JZ & McHughen A (1993) An improved procedure for production of transgenic flax plants usingAgrobacterium tumefaciens. Plant Sci. 88: 61–71
Gielen JJL, de Haan P, Kool AJ, Peters D, van Grinsven MQJM & Goldbach RW (1991) Engineered resistance to tomato spotted wilt virus, a negative-strand RNA virus. Bio/Technol. 9: 1363–1367
Halevy AH & Kofranek AM (1984) Evaluation of Lisianthus as a new flower crop. HortSci. 19: 845–847
Handa T (1992) Regeneration and characterization of prairie gentian (Eustoma grandiflorum) plant transformed byAgrobacterium rhizogenes. Plant Tiss. Cult. Lett. 9: 10–14
Hood EE, Helmer GL, Fraley RT & Chilton M (1986) The hypervirulence ofAgrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacter. 186: 1291–1301
Janssen B & Gardner RC (1989) Localized transient expression of GUS in leaf discs following cocultivation withAgrobacterium. Plant Molec. Biol. 14: 61–72
Jefferson RA, Kavanagh TA & Bevan MW (1987) GUS fusion:β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907
Klimyuk VI, Carrol BJ, Thomas CM & Jones GDJ (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. The Plant J. 3: 493–494
Li XQ, Liu CN, Ritchie SW, Peng JY, Gelvin SB & Hodges TK (1992) Factors influencingAgrobacterium-mediated transient expression ofgusA in rice. Plant Molec. Biol. 20: 1037–1048
Lisa V, Vaira AM, Milne RG, Luisoni E & Rapetti S (1990) Tomato spotted wilt virus in cinque specie coltivate in Liguria. Inf. Fitopatologico 12: 34–41
Matzke & Matzke (1995) How and why do plant inactivate homologous (trans)genes? Plant Physiol. 107: 679–685
McDonnell RE, Clark RD, Smith WA & Hinchee MA (1987) A simplified method for the detection of neomycin phosphotransferase II activity in transformed plant tissues. Plant Molec. Biol. Rep. 5: 380–386
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Pang SZ, Nagpala P, Wang M, Slightom JL & Gonsalves D (1992) Resistance to heterologous isolates of tomato spotted wilt virus in transgenic tobacco expressing its nucleocapsid protein gene. Phytopathol. 82: 1223–1229
Peterson GL (1977) A simplification of the protein assay method of Lowry et. al. which is more generally applicable. Annal. Bioch. 83:346–356
Ruffoni B, Damiano C, Massabò F & Esposito P (1990) Organogenesis and embryogenesis inLisianthus russelianus Hook. Acta Hortic. 280: 83–88
Ruffoni B, Giovannini A, Semeria L, Massabò F, Costantino C & Allavena A (1993) Tissue culture in lisianthus (Eustoma grandiflorum). In: Schiva T & Mercuri A (eds) Creating Genetic Variation in Ornamentals (pp. 289–296)
Sambrook J, Fritsch EF & Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press. Vol. 3 (p. A1)
Semeniuk P & Griesbach JR (1987)In vitro propagation of prairie gentian. Plant Cell, Tissue and Organ Culture 8: 249–253
Semeria L & Allavena A (1993) Genetic transformation ofEustoma grandiflorum Griseb. In: Schiva T & Mercuri A (eds) Creating Genetic Variation in Ornamentals (pp. 247–250)
Semeria L, Vaira AM, Accotto GP, & Allavena A (1995) Genetic transformation ofEustoma grandiflorum Griseb. by microprojectile bombardment. Euphytica 85: 125–130
Vaira AM, Semeria L, Crespi S, Lisa V, Allavena A & Accotto GP (1995) Resistance to tospoviruses inNicotiana benthamiana transformed with the N gene of tomato spotted wilt virus: correlation between transgene expression and protection in primary transformants. Mol. Plant Microb. Int. 8: 66–73
Vaira AM, Vecchiati M, Masenga V & Accotto GP (1996) A polyclonal antiserum against a recombinant viral protein combine specificity with versatility. J. Virol. Methods 56: 209–219
Wu FS & Wang MY (1984) Extraction of protein for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissue. Anal. Biochem. 139: 100–103
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Semeria, L., Ruffoni, B., Rabaglio, M. et al. Genetic transformation ofEustoma grandiflorum byAgrobacterium tumefaciens . Plant Cell Tiss Organ Cult 47, 67–72 (1996). https://doi.org/10.1007/BF02318967
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02318967