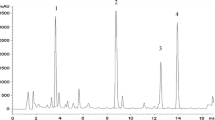
Abstract
Legume callus cultures were examined to assess whether regulation of phytoalexin biosynthetic pathways is retained in cultured tissues. Callus tissue cultures ofCanavalia ensiformis (jackbean),Medicago sativa (alfalfa), and nine species ofTrifolium (clover) were established (six clover species for the first time) and maintained on modified Gamborg's B5 medium. Phytoalexins educed in cultures incubated for 48 h with an abiotic elicitor (3.15 mM HgCl2) were detected by their antifungal activity and were purified by column chromatography and high-performance liquid chromatography. Following crystallization, phytoalexins were identified by ultraviolet and proton nuclear magnetic resonance spectroscopy. None of the treated cultures yielded the same complement of phytoalexins reported for fungal-inoculated leaves of the corresponding plants. Callus from all species exceptT. pratense yielded medicarpin, the only phytoalexin reported in treated leaves of all the corresponding plants. A second phytoalexin, maackiain, was found in treatedT. pratense andT. medium calli; maackiain has been reported in fungal-inoculated leaves of those plant species as well asT. hybridum. The phytoalexins sativan and vestitol were not found in treated callus tissues even though they were reported to be present in fungal-inoculated leaves of the same species. These results suggest that (a) the pathway for medicarpin biosynthesis is of central importance for this group of legumes, (b) some phytoalexin anabolic pathways contain metabolic blocks in cells of cultured tissue, and (c) the mechanism for regulating phytoalexin accumulation in tissues is not lost in culture.
Similar content being viewed by others
References
Butcher DN (1977) Secondary products in tissue cultures. In: Reinert J, Bajaj YPS (eds) Plant cell, tissue and organ culture. Berlin: Springer-Verlag, pp 668–693
Cline K, Albersheim P (1981) Host-pathogen interactions. XVII. Hydrolysis of biologically active fungal glucans by enzymes isolated from soybean cells. Plant Physiol 68:221–228
Dewick PM (1977) Biosynthesis of pterocarpan phytoalexins inTrifolium pratense. Phytochemistry 16:93–97
Dewick PM, Martin M (1979) Biosynthesis of pterocarpan and isoflavan phytoalexins inMedicago sativa: the biochemical interconversions of pterocarpans and 2′-hydroisoflavans. Phytochemistry 18:591–596
Ebel J, Ayers AR, Albersheim P (1976) Host-pathogen interactions. XII. Response of suspension-cultured soybean cells to the elicitor isolated fromPhytophthora megasperma var. sojae, a fungal pathogen of soybeans. Plant physiol 57:775–779
Gamborg OL (1970) The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol 45:372–375
Gustine DL (1981) Evidence for sulfhydryl involvement in regulation of phytoalexin accumulation inTrifolium repens callus tissue cultures. Plant Physiol 68: 1323–1326
Gustine DL, Sherwood RT, Vance CP (1978) Regulation of phytoalexin synthesis in jackbean callus cultures: stimulation of phenylalanine ammonia-lyase ando-methyltransferase. Plant Physiol 61:226–230
Hahlbrock K, Grisebach H (1975) Biosynthesis of flavonoids. In: Harbone JB, Mabry TJ, Mabry H (eds) The flavonoids, vol 2. New York: Academic Press, pp 866–915
Hargreaves JA, Mansfield JW, Coxon DT (1976) Identification of medicarpin as a phytoalexin in the broad bean plant (Vicia Faba L.). Nature 262:318–319
Higgins VJ, Smith DG (1972) Separtion and identification of two pterocarpanoid phytoalexins produced by red clover leaves. Phytopathology 62:235–238
Ingham JL (1976) Fungal modification of pterocarpan phytoalexins fromMelilotus alba andTrifolium pratense. Phytopathology 15:1489–1495
Ingham JL (1978) Isoflavonoid and stilbene phytoalexins of the genusTrifolium. Biochem Syst Ecol 6:217–223
Ingham JL (1979) Isoflavonoid phytoalexins of the genusMedicago. Biochem Syst Ecol 7:29–34
Ingham JL, Harborne JB (1976) Phytoalexin induction as a new dynamic approach to the study of systematic relationships among higher plants. Nature 260:241–242
Ingham JL, Millar RL (1973) Sativin: an induced isoflavan from the leaves ofMedicago sativa L. Nature 242:125–126
Keen NT (1972) Accumulation of wyerone in broadbean and demethylhomopterocarpin in jack bean after inoculation withPhytophthora megasperma var. sojae. Phytopathology 62:1365–1366
Keen NT, Sims JJ, Erwin DC, Rice E, Partridge JE (1971) 6a-Hydroxyphaseollin: an antifungal chemical induced in soybean hypocotyls byPhytophthora megasperma var. sojae. Phytopathology 61:1084–1089
Martin M, Dewick PM (1980) Biosynthesis of pterocarpan isoflavan and coumestan metabolites ofMedicago sativa: the role of an isoflav-3-ene. Phytochemistry 19: 2341–2346
Pachler KGR, Underwood WGE (1967) A proton magnetic resonance study of some pterocarpan derivatives. The conformation of the 6a, 11a-dihydro-6H-benzofuro [3, 2-c] [1] benzopyran ring system. Tetrahedron 23: 1817–1826
Smith DG, McInnes AG, Higgins VJ, Millar RL (1971) Nature of the phytoalexin produced by alfalfa in reponse to fungal infection. Physiol Plant Pathol 1:41–44
Yoshikawa M (1978) Diverse modes of action of biotic and abiotic phytoalexin elicitors. Nature 275:546–547
Author information
Authors and Affiliations
Additional information
Contribution no 8113 of the US Regional Pasture Research Laboratory, USDA-ARS, University Park, PA, USA
Rights and permissions
About this article
Cite this article
Gustine, D.L., Moyer, B.G. Retention of phytoalexin regulation in legume callus cultures. Plant Cell Tiss Organ Cult 1, 255–263 (1981). https://doi.org/10.1007/BF02318922
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02318922