Abstract
Background: Apoptosis (“programmed cell death”) is an active process characterized by prominent nuclear changes and DNA cleavage, which distinguishes it from cellular necrosis. In this study we investigated whether tamoxifen (TAM) treatment of estrogen receptor ER(+) MCF-7 and ER(-) MDA-231 human breast cancer cells resulted in cytotoxicity and cellular changes typical of apoptosis.
Methods: Cytotoxicity was measured using a tetrazolium assay. Cellular morphologic changes were observed using transmission electron microscopy. DNA cleavage was assessed using 1.6% agarose gel electrophoresis and was also quantitated biochemically.
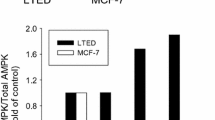
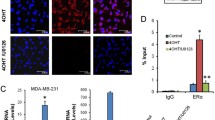
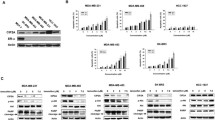
Results: Exposure of cells to TAM for 24 h resulted in dose- dependent cytotoxicity, and MCF-7 cells were somewhat more sensitive to TAM. TAM induced chromatin condensation around the nuclear periphery in both cell lines, changes typical of apoptosis. TAM-induced cytotoxicity correlated with dose-dependent DNA cleavage, which showed the characteristic “internucleosomal ladder.” DNA cleavage occurred at a slightly lower TAM dose and occurred somewhat sooner in MCF-7 cells. TAM-induced DNA cleavage in MCF-7 cells was inhibited by the protein synthesis inhibitor cycloheximide, the RNA synthesis inhibitor actinomycin D, and by 17β-estradiol. However, in MDA-231 cells, DNA cleavage was inhibited by cycloheximide, partially but not significantly inhibited by actinomycin D, and not inhibited by 17β-estradiol.
Conclusions: TAM induces typical apoptosis in ER(+) or ER (-) human breast cancer cells. TAM induction of apoptosis in MCF-7 cells involves the estrogen receptor, and requires the synthesis of new protein and mRNA. TAM induction of apoptosis in MDA-231 cells depends primarily on protein synthesis. TAM-induced cytotoxicity and DNA damage appear to be explained in part by the induction of apoptosis.
Similar content being viewed by others
References
Love RR. Tamoxifen therapy in primary breast cancer: biology, efficacy, and side effects.J Clin Oncol 1989;7:803–815.
Goldenberg GJ, Froses EK. Drug and hormone sensitivity of estrogen receptor-positive and -negative human breast cancer cells in vitro.Cancer Res 1982;42:5147–51.
Taylor CM, Blanchard B, Zava DT. Estrogen receptor-mediated and cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen.Cancer Res 1984;44:1409–14.
Osborne CK, Boldt DH, Clark GM, Trent JM. Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase.Cancer Res 1983;43:3583–5.
Reddel RR, Murphy LC, Hall RE, Sutherland RL. Differential sensitivity of human breast cancer cell lines to the growth-inhibitory effects of tamoxifen.Cancer Res 1985;45:1525–31.
Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics.Br J Cancer 1972;26:239–57.
Arends MJ, Morris RG, Wyllie AH. Apoptosis: the role of the endonuclease.Am J Pathol 1990;136:593–608.
Owens GP, Hahn WE, Cohen JJ. Identification of mRNAs associated with programmed cell death in immature thymocytes.Mol Cell Biol 1991;11:4177–88.
Ling YH, Priebe W, Perez-Soler R. Apoptosis induced by anthracycline antibiotics in P388 parent and multidrugresistant cells.Cancer Res 1993;53:1845–52.
Evans DL, Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes.Cancer Res 1993;53:2133–9.
Walton MI, Whysong D, O'Connor PM, Hockenbery D, Korsmeyer SJ, Kohn KW. Constitutive expression of humanbcl-2 modulates nitrogen mustard and camptothecin induced apoptosis.Cancer Res 1993;53:1853–61.
Kaufman S. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other anticancer drugs: a cautionary note.Cancer Res 49;5870–8.
Rotello RJ, Lieberman RC, Purchio AF, Gerscgebson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor β1 in cultured uterine epithelial cells.Proc Natl Acad Sci USA 1991;88:3412–5.
Shinagawa T, Yoshioka K, Kakumu S, Wakita T, Ishikawa T, Itoh Y, Takayangai M. Apoptosis in cultured rat hepatocytes: the effects of tumour necrosis factor α and interferon γ.J Pathol 1991;165:247–53.
Schwartzman RA, Cidlowski JA. Mechanism of tissuespecific induction of internucleosomal deoxyribonucleic acid cleavage activity and apoptosis by glucocorticoids.Endocrinology 1993;133:591–9.
Kyprianou N, English HF, Isaacs JT. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation.Cancer Res 1990;50:3748–53.
Szende B, Zalatnai A, Schally AV. Programmed cell death (apoptosis) in pancreatic cancers of hamsters after treatment with analogs of both luteinizing hormone-releasing hormone and somatostatin.Proc Natl Acad Sci USA 1989;86:1643–7.
Lippman ME, Bolan G, Huff KK. The effects of estrogens and antiestrogens on hormone responsive human breast cancer in long term tissue cultures.Cancer Res 1976;36:4595–601.
Clemmons DR, Camacho-Hubner C, Coronado E, Osborne CK. Insulin-like growth factor binding protein secretion by breast carcinoma cell lines: correlation with estrogen receptor status.Endocrinology 1990;127:2679–86.
Reddel RR, Murphy LC, Sutherland RL. Factors affecting the sensitivity of T-47D human breast cancer cells to tamoxifen.Cancer Res 1984;44:2398–405.
Carmichael J, DeGraff WG, Gadzar AF, Minna J, Mitchell JB. Evaluation of a tetrazolium based semiautomated colorimetric assay: assessment of chemosensitivity testing.Cancer Res 1987;47:936–42.
Kang Y, Perry RR. Modulatory effects of tamoxifen and recombinant human α-interferon on doxorubicin resistance.Cancer Res 1993;53:3040–5.
McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate an endogenous suicide process in thymocytes through the elevation of cytosolic Ca2+ concentration.Arch Biochem Biophys 1989;269:365–70.
Tilly JL, Billig H, Kowalski KI, Hsueh AJ. Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism.Mol Endocrinol 1992;6:1942–50.
Owens GP, Cohen JJ. Identification of genes involved in programmed cell death.Cancer Metastasis Rev 1992;11:149–156.
Gorczyca W, Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents.Cancer Res 1993;53:3186–92.
Dive C, Hickman JA. Drug-target interactions: only the first step in the commitment to a programmed cell death?Br J Cancer 1991;64:192–6.
Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts byc-myc protein.Cell 1992;69:119–28.
Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line.Proc Natl Acad Sci USA 1992;89:4495–4499.
Miyashita T, Reed JC.Bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs.Cancer Res 1992;52:5407–11.
Fanidi A, Harrington EA, Evan GI. Cooperative interaction betweenc-myc andbcl-2 proto-oncogenes.Nature 1992;359:554–6.
Kirk J, Houlbrook S, Stuard NSA, Stratford IJ, Harris AL, Carmichael J. Differential modulation of doxorubicin toxicity to multidrug and intrinsically drug resistant cell lines by anti-oestrogens and their major metabolites.Br J Cancer 1993;67:1189–95.
Scambia G, Ranelletti FO, Panici PB, et al. Synergistic antiproliferative activity of tamoxifen and cisplatin on primary ovarian tumours.Eur J Cancer 1992;28A:1885–9.
Bardon S, Vignon F, Montcourrier P, Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells.Cancer Res 1987;47:1441–8.
Warri AM, Huovinen RL, Laine AM, Martikainen PM, Harkonen PL. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro.J Natl Cancer Inst 1993;85:1412–8.
Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB. Evidence that transforming growth factor-β is a hormonally regulated negative growth factor in human breast cancer cells.Cell 1987;48:417–28.
Zugmaier G, Lippman ME. Effects of TGF-β on normal and malignant mammary epithelium.Ann NY Acad Sci 1990;593:272–5.
Taetle R, Payne C, Dos Santos B, Russell M, Segarini P. Effects of transforming growth factor 1 on growth and apoptosis of human acute myelogenous leukemia cells.Cancer Res 1993;53:3386–93.
Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment.Cancer Res 1989;49:2175–83.
Stuart NSA, Philip P, Harris AL, et al. High-dose tamoxifen as an enhancer of etoposide cytotoxicity: clinical effects and in vitro assessment in p-glycoprotein expressing cell lines.Br J Cancer 1992;27:833–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perry, R.R., Kang, Y. & Greaves, B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Annals of Surgical Oncology 2, 238–245 (1995). https://doi.org/10.1007/BF02307030
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02307030