Abstract
Background: Transfection of the costimulatory molecule B7-1 into some murine tumors can increase antitumor immunity and eradicate tumor growth. The purpose of this work was to construct an adenovirus-B7 (Ad-B7) expression vector and study B7-1 gene transfer into human cancer cells.
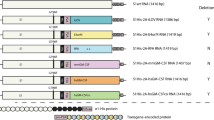
Methods: The human B7-1 cDNA was ligated into an expression cassette containing the human cytomegalovirus immediate early gene promoter and then inserted into the E1 region of the Ad5 genome by homologous recombination. The resulting Ad-B7 vector was used to infect established cancer cell lines and freshly resected cancers. Resected tumors were disaggregated into single cell suspensions by mechanical mincing and enzymatic digestion. Surface expression of B7-1 after infection was verified by flow cytometry.
Results: Expression kinetics in three cell lines showed that infected cells began to express B7-1 within 24 h. The proportion of B7-1+ cells continued to increase during the next 48 h, after which expression remained relatively constant during the next 5 days (up to 98% B7-1+ cells). Fresh tumor cells from various cancers displayed similar kinetics, but with greater variability in the proportion of cells expressing B7-1 (13% to 95% B7-1+ cells). Cancers which were successfully infected included 3 colorectal adenocarcinomas, 2 leiomyosarcomas, 2 lung squamous cell carcinomas, and 1 renal cell carcinoma.
Conclusions: The Ad-B7 vector is a rapid and efficient means of gene transfer which does not require host cell proliferation. The ultimate objective is to engineer autologous tumors to express B7-1 and vaccinate cancer patients in an adjuvant or palliative setting.
Similar content being viewed by others
References
Schwartz RH. A cell culture model for T lymphocyte clonal anergy.Science 1990;248:1349–56.
Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy.Annu Rev Immunol 1989;7:445–80.
Liu Y, Linsley PS. Costimulation of T cell growth.Curr Opin Immunol 1992;4:265–70.
Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2.PNAS 1991;88:6575–9.
Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells.J Immunol 1991;147:2461–6.
Freeman GJ, Freeman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells.J Immunol 1987;143:2714–22.
Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou L-J, White M, Fingeroth JD, et al. Structure, expression and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7.J Exp Med 1991;174:625–31.
Chen L, Ashe S, Brady WA, Hellström I, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4.Cell 1992;71:1093–102.
Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells.Science 1993;259:368–70.
Dessureault S, Gallinger S. Lymphocyte responses to B7 expressing human cancer cell lines.J Immunother 1994;16:248.
Berkner KL. Development of adenovirus vectors for expression of heterologous genes.Biotechniques 1988;6:616–29.
Prevec L, Schneider M, Rosenthal KL, Belbeck LW, Derbyshire JB, Graham FL. Use of human adenovirus-based vectors for antigen expression in animals.J Gen Virol 1989;70;429–34.
Graham FL. Adenoviruses as expression vectors and recombinant vaccines.Trends Biotechnol 1990;8;85–7.
Levrero M, Barban V, Manteca S, Ballay A, Balsamo C, Avantaggiati MT, Natoli G, et al. Defective and nondefective adenovirus vector for expressing foreign genes in vitro and in vivo.Gene 1991;101:195–201.
Graham FL, Prevec L. Manipulation of adenovirus vectors. In: Murray EJ, Walker JM, eds.Methods in molecular biology (Vol 7: Gene transfer and expression protocols). Clifton, New Jersey: Humana Press, 1991:109–28.
Graham FL, Prevec L. Adenovirus expression vectors and recombinant vaccines. In: Ellis RW, ed.Vaccines; new approaches to immunological problems. Boston: Butterworth-Heinemann, 1992;364–90.
Haddada H, Ragot T, Cordier L, Duffour MT, Perricaudet M. Adenoviral interleukin-2 gene transfer into P815 tumor cells abrogates tumorigenicity and induces antitumoral immunity in mice.Human Gene Ther 1993;4;703–11.
Braciak TA, Mittal SK, Graham FL, Richards CD, Gauldie J. Construction of recombinant human type 5 adenoviruses expressing rodent IL-6 genes: an approach to investigate in vivo cytokine function.J Immunol 1993;151;5145–53.
Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3.PNAS 1994;91:8802–6.
Howard RB, Shroyer KR, Marcell T, Swanson LE, et al. Time-related effects of enzymatic disaggregation on model human lung carcinomas.Cytometry 1995;19:146–53.
Riedy MC, Muirhead KA, Jensen CP, Stewart CC. Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell populations.Cytometry 1991;12:133–9.
Stewart CC. Clinical applications of flow cytometry.Cancer 1992;69:1543–52.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dessureault, S., Graham, F. & Gallinger, S. B7-1 gene transfer into human cancer cells by infection with an adenovirus-B7 (Ad-B7) expression vector. Annals of Surgical Oncology 3, 317–324 (1996). https://doi.org/10.1007/BF02306289
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02306289