Abstract
Background: Alternative medicines are frequently used by patients with breast cancer for general health benefits. American ginseng, an herbal remedy, purportedly alleviates treatment-induced postmenopausal symptoms.
Methods: Estrogenic potential of American ginseng root extract to induce the expression of pS2, an estrogen-regulated gene, was evaluated in breast cancer cell lines MCF-7, T-47D, and BT-20 by Northern and Western blot analysis. Competitive studies were performed with ginseng in combination with tamoxifen. Cell proliferation assays were performed using the tetrazolium dye procedure and direct cell count.
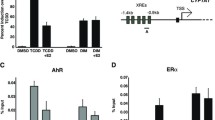
Results: Ginseng and estradiol induce the expression of pS2 RNA and protein in MCF-7 cells, whereas tamoxifen suppresses expression. Neither ginseng nor estradiol induced increased pS2 expression in T-47D or BT-20 cell lines. Although estradiol exhibited a proliferative effect and tamoxifen had an inhibitory effect, ginseng demonstrated no significant effect on cell proliferation.
Conclusions: The results of this study suggest that ginseng may exhibit estrogenlike effects on estrogen receptor-positive breast cancer cells by inducing pS2 expression and that the effect of ginseng may be mediated in part through the estrogen receptor. Because ginseng does not exhibit a proliferative effect, it may play a protective role against breast cancer rather than serve as a mitogen.
Similar content being viewed by others
References
Zhou DH. Preventive geriatrics: an overview from traditional Chinese medicine.Am J Chin Med 1982;10:32–9.
Hu SY. A contribution to our knowledge of ginseng.Am J Chin Med 1977;5:1–23.
Li CP, Li RC. An introductory note to ginseng.Am J Clin Med 1973;1:249–61.
Kessel B, Duda RB, Prouty J, et al. The use of antioxidants and unconventional alternatives to estrogen in postmenopausal women with and without breast cancer [Abstract S-8]. Presented at The North American Menopause Society 5th Annual Meeting, Washington, DC, September 22–24, 1994.
Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States.N Engl J Med 1993;328:246–52.
Hu SY. A contribution to our knowledge of ginseng.Am J Chin Med 1977;5:1–23.
Fugimoto Y, Satoh M, Takeuchi N, Kirisawa M. Cytotoxic acetylenes from Panax quinquefolium.Chem Pharm Bull 1991;39:521–3.
Hopkins MP, Androff L, Benninghoff AS. Ginseng face cream and unexplained vaginal bleeding.Am J Obstet Gynecol 1988;159:1121–2.
Arkko PJ, Arkko BL, Kari-Koskinen O, Taskinen PJ. A survey of unproven cancer remedies and their uses in an outpatient clinic for cancer therapy in Finland.Soc Sci Med 1980;14A:511–4.
Downer SM, Cody MM, McCluskey P, et al. Pursuit and practice of complementary therapies by cancer patients receiving conventional treatment.Br Med J 1994;309:86–9.
Brown AMC, Jeltsch JM, Roberts M, Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line.Proc Natl Acad Sci U S A 1984;81:6344–8.
Nunez AM, Jakowlev S, Briand JP, et al. Characterization of the estrogen-induced pS2 protein secreted by the human breast cancer cell line MCF-7.Endocrinology 1987;121:1759–65.
Westley B, May FEB, Brown AMC, et al. Effects of antiestrogens on the estrogen-regulated pS2 RNA and the 52- and 160-kilodalton proteins in MCF7 cells and two tamoxifen-resistant sublines.J Biol Chem 1984;259:10030–5.
Sathyamoorthy N, Wang TTY, Phang JM. Stimulation of pS2 expression by diet-derived compounds.Cancer Res 1994;54:957–61.
Alvarez JG, Touchstone JC, Storey BT, Grob RL. Determination of sphingolipid sphingoid bases by HPTLC-fluorescence spectrodensitometry.J Liquid Chromatogr 1990;12:3115–9.
Alvarez JG, Touchstone JC. Separation of acidic and neutral lipids by aminopropyl-bonded silica gel column chromatography.J Chromatogr 1992;577:142–5.
Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. Steroid receptor analyses of nine human breast cancer cell lines.Cancer Res 1978;38:2434–7.
Horwitz KB, McGuire WL. Estrogen control of progesterone receptor in human breast cancer.J Biol Chem 1978;253:2223–8.
Chirgwin JM, Przbyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease.Biochemistry 1979;18:5294–9.
Ausubel FM, Brent R, Kingston RE, et al.Current protocols in molecular biology. New York: John Wiley & Sons, 1989.
Sambrook J, Fritsch EF, Maiatis T.Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1989.
Camby I, Kiss R. In vitro estradol-sensitivity characterization of the MCF-7, ZR-75, MDA-MB-231 and T-47D human breast neoplastic cell lines.Anticancer Res 1993;13:2355–60.
Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability.J Immunol Methods 1986;89:271–7.
Carmichael J, De Graff WG, Gazdar AF, et al. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing.Cancer Res 1987;47:936–42.
Liu CX, Xiao PG. Recent advances on ginseng research in China.J Ethnopharmacol 1992;36:27–38.
Lee KD, Huemer RP. Antitumoral activity of panax ginseng extracts.Jpn J Pharmacol 1971;21:299–301.
Yun YS, Lee YS, Jo SK, Jung IS. Inhibition of autochthonous tumor by ethanol insoluble fraction from panax ginseng as an immunomodulator.Planta Med 1993;59:521–4.
Kim JY, Germolec DR, Luster MI. Panax ginseng as a potential immunomodulator: studies in mice.Immunopharmacol Immunotoxicol 1990;12:257–76.
Rhee YH, Ahn JH, Choe J, Kang KW, Joe C. Inhibition of mutagenesis and transformation by root extracts of panax ginseng in vitro.Planta Med 1991;57:125–8.
Willett WC. Micronutrients and cancer risks.Am J Clin Nutr 1994;59:1162S-5S.
Greenwald P. Strengths and limitations of methodologic approaches to the study of diet and cancer: summary and future perspectives with emphasis on dietary fat and breast cancer.Prev Med 1989;18:163–6.
Yu H, Harris RE, Gao YT, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shangai, China versus the United States.Int J Epidemiol 1991;20:76–81.
Rose DP. Dietary fiber, phytoestrogens, and breast cancer.Nutrition 1992;8:47–51.
Adlercruetz H, Hockerstedt K, Bannwart C, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG).J Steroid Biochem 1987;27:1135–44.
Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavenoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet.Am J Clin Nutr 1991;54:1093–100.
Adlercreutz H, Mousavi Y, Clark J, et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies.J Steroid Biochem Mol Biol 1992;41:331–7.
Adlercreutz H, Bannwart C, Wahala K, et al. Inhibition of human aromatase by mammalian lignans and isoflavenoid phytoestrogens.J Steroid Biochem Mol Biol 1993;44:147–53.
Foekens JA, Rio MC, Seguin P, et al. Prediction of relapse and survival in breast cancer patients by pS2 protein status.Cancer Res 1990;50:3832–7.
Thompson AM, Hawkins RA, Elton RA, Steel CM, Chetty U, Carter DC. pS2 is an independent factor of good prognosis in primary breast cancer.Br J Cancer 1993;68:93–6.
Schwartz LH, Koerner FC, Edgerton SM, et al. pS2 espression and response to hormonal therapy in patients with advanced breast cancer.Cancer Res 1991;51:624–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duda, R.B., Taback, B., Kessel, B. et al. pS2 expression induced by American ginseng in MCF-7 breast cancer cells. Annals of Surgical Oncology 3, 515–520 (1996). https://doi.org/10.1007/BF02306082
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02306082