Abstract
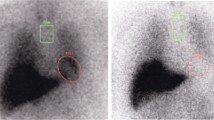
Scintigraphy with radiolabeled metaiodobenzylguanidine (MIBG) enables the visualization and quantification of functionally intact adrenergic neurons and cells. In Parkinson disease, MIBG uptake of postganglionic cardiac sympathetic neurons is grossly reduced at an early stage of the disease in almost all patients with a clinical severity score of Hoehn and Yahr II or higher. Based on the meta-analysis of studies with a total of 246 cases of Parkinson disease and 45 cases of multiple system atrophy, the overall sensitivity to positively identify patients with Parkinson disease was 89.7%, and the specificity to discriminate them from patients with multiple system atrophy was 94.6%. Quantification of cardiac MIBG uptake is a valuable tool to identify patients with Parkinson disaase and to discriminate them from other neurodegenerative disorders early in the course of the disease.
Similar content being viewed by others
References
Wieland DM, Brown LE, Tobes MC. Imaging the primate adrenal medulla with 1231 and 1311 meta-iodobenzylguanidine.J Nucl Med 1981; 22:358–364.
Solanki KK, Bomanji J, Moyes J, et al. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG).Nucl Med Commun 1992; 13:513–521.
Wafelman AR, Hoefnagel CA, Maes RA, et al. Radioiodinated metaiodobenzylguanidine: a review of its biodistribution and pharmacokinetics, drug interactions, cytotoxicity and dosimetry.Eur J Nucl Med 1994; 21:545–559.
Glowniak JV, Kilty JE, Amara SG, et al. Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters.J Nucl Med 1993; 34:114–116.
Jaques S, Tobes MC, Sisson JC. Sodium dependency of uptake of norepinephrine andm-iodobenzylquanidine into cultured human pheochromocytoma cells: evidence of uptake-one.Cancer Res 1987; 47:3920–3928.
Wieland DM, Mangner TJ, Inbasekaran MN, et al. Adrenal medulla imaging agents: a structure-distribution relationship study of radiolabeled aralkylguanidines.J Med Chem 1984; 27:149–155.
Gasnier B, Roisin MP, Scherman D, et al. Uptake of metaiodobenzylguanidine by bovine chromaffin granule membranes.Mol Pharmacol 1986; 29:275–280.
Sisson JC, Wieland DM, Sherman P, et al. Metaiodobenzylguanidine as an index of the adrenergic nervous system integrity and function.J Nucl Med 1987; 28:1620–1624.
Sisson JC, Shapiro B, Meyers L, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man.J Nucl Med 1987; 28:1625–1636.
Jaques S, Tobes MC. Comparison of the secretory mechanisms of meta-iodobenzylguanidine (MIBG) and norepinephrine (NE) from the cultured bovine adrenomedullary cells [abstract].J Nucl Med 1985; 26:17.
Sisson JC, Lynch JJ, Johnson J, et al. Scintigraphic detection of regional disruption of adrenergic neurons in the heart.Am Heart J 1988; 116:67–76.
Dae MW, O'Connell JW, Botvinick EH, et al. Scintigraphic assessment of regional cardiac adrenergic innervation.Circulation 1989; 79:634–644.
Satoh A, Serita T, Seto M, et al. Loss of123I-MIBG uptake by the heart in Parkinson's disease: assessment of cardiac denervation and diagnostic value.J Nucl Med 1999; 40:371–375.
Yoshita M. Differentiation of idiopathic Parkinson's disease from striatonigral degeneration and progressive supranuclear palsy using iodine 123 metaiodobenzyl-guanidine myocardial scintigraphy.J Neurol Sci 1998; 155:60–67.
Taki J, Nakajima K, Hwang EH, et al. Peripheral sympathetic dysfunction in patients with Parkinson's disease without autonomic failure is heart selective and disease specific.Eur J Nucl Med 2000; 27:566–573.
Iwasa K, Nakalima K, Yoshikawa H, et al. Decreased myocardial 123I-MIBG uptake in Parkinson's disease.Acta Neurol Scand 1998; 97:303–336.
Mathias CJ, Senard JM, Braune S, et al. L-threo-dihydroxyphenylserine (L-threo-DOPS; droxidopa) in the management of neurogenic orthostatic hypotension: a multi-national, multi-centre dose ranging study in multiple system atrophy (MSA) and pure autonomic failure (PAF).Clin Auton Res 2001; 11:235–242.
Yoshita M, Braune S. Cardiac uptake of [123I]MIBG separates PD from multiple system atrophy.Neurology 2000; 54:1877–1878.
Ando Y, Obayashi K, Tanaka Y, et al. Radiolabelled metaiodobenzylguanidine in assessment of autonomic dysfunction.Lancet 1994; 343:984–985.
Hakusui S, Yasuda T, Yanagi T, et al. [123I-MIBG myocardial scintigraphical analysis in patients with and without autonomic disorder].Rinsho Shinkeigaku 1994; 34:402–404 [in Japanese].
Langer A, Freeman MR, Josse RG, et al. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia.J Am Coll Cardiol 1995; 25:610–618.
Kreiner G, Wolzt M, Fasching P, et al. Myocardial m-[123I]iodobenzylguanidine scintigraphy for the assessment of adrenergic cardiac innervation in patients with IDDM: comparison with cardiovascular reflex tests and relationship to left ventricular function.Diabetes 1995; 44:543–549.
Schnell O, Kirsch CM, Stemplinger J, et al. Scintigraphic evidence for cardiac sympathetic dysinnervation in long-term IDDM patients with and without ECG-based autonomic neuropathy.Diabetologia 1995; 38:1345–1352.
Senard JM, Valet P, Durrieu G, et al. Adrenergic supersensitivity in parkinsonians with orthostatic hypotension.Eur J Clin Invest 1990; 20:613–619.
Braune S, Reinhardt M, Bathmann J, et al. Impaired cardiac uptake of meta-[123I]iodobenzylguanidine in Parkinson's disease with autonomic failure.Acta Neurol Scand 1998; 97:307–314.
Reinhardt MJ, Jüngling FD, Krause TM, et al. Scintigraphic differentiation between two forms of primary dysautonomia early after onset of autonomic dysfunction: value of cardiac and pulmonary123I-MIBG uptake.Eur J Nucl Med 2000; 27:595–600.
Goldstein DS, Holmes C, Li ST, et al. Cardiac sympathetic denervation in Parkinson disease.Ann Intern Med 2000; 133:338–347.
Takatsu H, Nishida H, Matsuo H, et al. Cardiac sympathetic denervation from the early stage of Parkinson's disease: clinical and experimental studies with radiolabeled MIBG.J Nucl Med 2000; 41:71–77.
Wakabayashi K, Takahashi H, Ohama E, et al. Lewy bodies in the visceral autonomic nervous system in Parkinson's disease.Adv Neurol 1993; 60:609–612.
Wakabayashi K. Parkinson's disease: the distribution of Lewy bodies in the peripheral autonomic nervous system.No To Shinkei 1989; 41:965–971 [English abstract].
Goldstein DS, Holmes C, Cannon R, et al. Sympathetic cardioneuropathy in dysautonomias.N Engl J Med 1997; 336:696–702.
Takatsu H, Nagashima K, Murase M, et al. Differentiating Parkinson's disease from multiple system atrophy by measuring cardiac iodine-123 metaiodobenzylguanidine accumulation.JAMA 2000; 284:44–45.
Orimo S, Ozawa E, Nakade S, et al. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson's disease.J Neurol Neurosurg Psychiatry 1999; 67:189–194.
Braune S, Reinhardt M, Schnitzer R, et al. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy.Neurology 1999; 53;1020–1025.
Druschky A, Hilz MJ, Platsch G, et al. Differentiation of Parkinson's disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT.J Neurol Sci 2000; 175:3–12.
Brooks DJ. Morphological and functional imaging studies on the diagnosis and progression of Parkinson's disease.J Neurol 2000; (Suppl 2):II/11–II/18.
Antonini A, Leenders KL, Vontobel P, et al. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson's disease.Brain 1997; 120:2187–2195.
Antonini A, Kazumata K, Feigin A, et al. Differential diagnosis of parkinsonism with [18F]fluorodeoxyglucose and PET.Mov Disord 1998; 13:268–274.
Brefel-Courbon C, Courbon F, Thalamas C, et al. Myocardial meta-[123I] iodobenzylguanidine (MIBG) uptake is different in Parkinson's disease and multiple system atrophy with autonomic failure [abstract].Clin Auton Res 1999; 9:285.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braune, S. The role of cardiac metaiodobenzylguanidine uptake in the differential diagnosis of parkinsonian syndromes. Clinical Autonomic Research 11, 351–355 (2001). https://doi.org/10.1007/BF02292766
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02292766