Summary
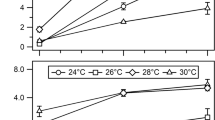
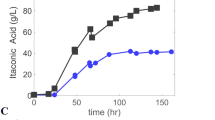
Kinetics of growth and nitrogenase induction inFrankia sp. Ar13 were studied in batch culture. Growth on defined medium with NH +4 as the N source displayed typical batch culture kinetics; however, a short stationary phase was followed by autolysis. Removal of NH +4 arrested growth and initiated vesicle differentiation. Vesicle numbers increased linearly and were paralleled by a rise in nitrogenase (acetylene reduction) activity. Nitrogenase activity (10 nM C2H4·mg protein−1·min−1) was sufficient to support growth on N2 and protein levels rose in parallel with nitrogenase induction. Optimal conditions for vesicle and nitrogenase induction were investigated. Maximum rates of acetylene reduction were obtained with 5 to 10 mM K2 HPO4/KH2PO4, 0.1 mM CaCl2 and MgSO4. The optimum pH for acetylene reduction and respiration was around 6.7. The amount (5 to 10 μg protein/ml) and stage (exponential) of growth of the ammonium-grown inoculum strongly influenced the subsequent development of nitrogenase activity. Propionate was the most effective carbon source tested for nitrogenase induction. Respiration in propionate-grown cells was stimulated by CO2 and biotin, suggesting that propionate is metabolized via the propionyl CoA pathway.
Similar content being viewed by others
References
Aguilar O M and Favelukes G 1982 Requirement for carbon dioxide for nonsymbiotic expression ofRhizobium japonicum nitrogenase activity. J. Bacteriol. 152, 510–513.
Akkermans A D L 1971 Nitrogen fixation and nodulation ofAlnus andHippophaë under natural conditions. Ph.D. Thesis, University of Leiden, The Netherlands.
Akkermans A D L, Roelofsen W, Blom J, Huss-Danell K and R Harkink 1983 Utilization of carbon and nitrogen compounds byFrankia in synthetic media and in root nodules ofAlnus glutinosa, Hippophae rhamnoides andDatisca cannabina. Can. J. Bot., 2793–2800.
Arias J M, Fernandez-Vivas A, Montoya E 1983 Evidence for an activating substance related to autolysis inMyxococcus coralloides D. Arch. Microbiol. 134, 164–166.
Baker D, Torrey J G and Kidd G H 1979 Isolation by sucrose-density fractionation and cultivationin vitro of actinomycetes from nitrogen-fixing root nodules. Nature London 281, 76–78.
Baker D and Torrey J G 1979 The isolation and cultivation of actinomycetous root nodule endophytes.In Symbiotic Nitrogen Fixation in the Management of Temperate Forests. pp 38–56. Eds. J C Gordon, C T Wheeler and D A Perry. Forest Research Laboratory, Oregon State University, Corvallis, OR.
Baker D, Newcomb W and Torrey J G 1980 Characterization of an ineffective actinorhizal microsymbiont,Frankia sp. Eu11 (Actinomycetales). Can. J. Microbiol. 26, 1072–1089.
Baker D 1982–1983 A cumulative listing of isolatedFrankia, the symbiotic nitrogen fixing actinomycetes. The Actinomycetes 17, 35–42.
Becking J H 1970 Frankiaceae fam. nov. (Actinomycetales) with new combination and six new species of the genusFrankia Brunchorst 1886. Int. J. Syst. Bacteriol. 20, 201–220.
Becking J H 1977 Endophyte and association establishment in non-leguminous nitrogen-fixing plants. In Recent Developments in Nitrogen Fixation, pp 551–567. Eds. W Newton, J R Postgate and C Rodriguez-Barrueco, Academic Press, London.
Benson D R and Hanna D 1983Frankia diversity in an alder stand as estimated by SDS-PAGE of whole cell proteins. Can J. Bot. 61, 2919–2923.
Berry A and Torrey J G 1979 Isolation and characterizationin vitro of an actinomycetous endophyte fromAlnus rubra Bong.In Symbiotic Nitrogen Fixation in the Management of Temperate Forests. pp 69–83. Eds J C Gordon, C T Wheeler and D A Perrry Oregon State Univ., Corvallis, OR.
Blom J, Roelofsen W and Akkermans A D L 1980 Growth ofFrankia AvcIl on media containing Tween 80 as C-source. FEMS Microbiol. Letters 9, 131–135.
Blom J 1981 Utilization of fatty acids and NH +4 byFrankia AvcIl. FEMS Microbiol. Letters 10, 143–145.
Blom J, Roelofsen W and Akkermans A D L 1981 Assimilation of nitrogen in root nodules of alder (Alnus glutinosa). New Phytol. 89, 321–326.
Boland M J, Farnden K J F and Robertson, J G 1980. Ammonia assimilation in nitrogenfixing legume nodules.In Nitrogen Fixation, Vol II. Eds. W E Newton and W H Orme-Johnson. Univ. Park Press, Baltimore.
Bradford M M 1976 A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72, 248–254.
Burggraaf A J P and Shipton W A 1982 Estimates ofFrankia growth under various pH and temperature regimes. Plant and Soil 69, 135–147.
Burggraaf A J P and Shipton W A 1983 Studies on the growth ofFrankia isolates in relation with infectivity and nitrogen fixation (acetylene reduction). Can. J. Bot. 61, 2774–2782.
Calam C T 1969 The evaluation of mycelial growth.In Methods in Microbiology, pp 567–591. Vol I. Eds. J R Norris and D W Ribbons. Academic Press, New York.
Callaham D, DelTredici P and Torrey J G 1978 Isolation and cultivationin vitro of the Actinomycete causing root nodulation inComptonia. Science 199, 899–902.
DeHertogh A A, Mayeux P A and Evans H J 1964 The relationship of cobalt requirement to propionate metabolism inRhizobium. J. Biol. Chem. 239, 2446–2453.
Dosôil J, Sikyta B, Kašparova J, Doskočilova D and Zajiček J 1958 Development of the culture ofStreptomyces rimosus in submerged fermentation. J. Gen. Microbiol. 18, 302–314.
Drews G 1965 Untersuchungen zur regulation der bacteriochlorophyll-synthese beiRhodospirillum rubrum. Arch. Mikrobiol. 51, 186–198.
Gauthier D, Diem H G and Dommergues Y 1981In vitro nitrogen fixation by two actinomycete strains isolated fromCasuarina nodules. Appl. Environ. Microbiol. 41, 306–308.
Giovanelli, J and Stumpf P F 1958 Fat metabolism in higher plants. X. Modified β-oxidation of propionate by peanut mitochondria. J. Biol. Chem. 231, 411–426.
Kretschmer S, Riesenberg D and Bergten F 1981 Comparative analysis of mycelial growth.In Actinomycetes, Zbl. Bakt. Suppl. 11, 131–135.
Lalonde M and Calvert H E 1979 Production ofFrankia hyphae and spores as an infective inoculation forAlnus species.In Symbiotic Nitrogen Fixation in the Management of Temperate Forests. pp 95–110. Eds. J C Gordon, C T Wheeler and D A Perry. Forest Research Laboratory, Oregon State Univ, Corvallis OR.
Lalonde M, Calvert H E, and Pine S 1981 Isolation and use ofFrankia strains in actinorhizae formation.In Current perspectives in Nitrogen Fixation pp 296–299. Eds. A Gibson and W Newton. Australian Academy of Science Canberra.
Lechevalier M and Lechevalier H A 1979 The taxonomic position of the Actinomycetic Endophytes.In Symbiotic Nitrogen Fixation in the Management of Temperate Forests. pp 111–122. Eds. J C Gordon, C T Wheeler and D A Perry Oregon State Univ, Corvallis OR.
Mian S and Bond G 1978 The onset of nitrogen fixation in young alder plants and its relation to differentiation in the nodular endophyte. New Phytol. 80, 187–192.
Newcomb W, Peterson R L, Callaham D and Torrey J G 1978 Structure and hostactinomycete interactions in developing root nodules ofComptonia peregrina. Can. J. Bot. 56, 502–531.
O'Gara F and Shanmugan K T 1976 Regulation of nitrogen fixation byRhizobium export of fixed N2 as NH +4 . Biochim Biophys. Acta 437, 313–321.
Postgate J R and Hunter J R 1964 Accelerated death ofAerobacter aerogenes starved in the presence of growth-limiting substrates. J. Gen. Microbiol. 34, 459–473.
Quispel A and Tak T 1978 Studies on the growth of the endophyte ofAlnus glutinosa (L.) Vill. New Phytol. 81, 587–600.
Shipton W A and Burggraaf A J P 1982 A comparison of the requirements for various carbon and nitrogen sources and vitamins in someFrankia isolates. Plant and Soil 69, 149–161.
Strange R E, Dark F A, and Ness A G 1961 The survival of stationary phaseAerobacter aerogenes spores in aqueous suspension. J. Gen. Microbiol. 25, 61–76.
Tisa L, McBride M and Ensign J C 1983 Studies of growth and morphology ofFrankia strains EANIpec, Eullc, CpII and ACNIAG. Can. J. Bot. 61, 2768–2773.
Tjepkema J D and Yocum C S 1973 Respiration and oxygen transport in soybean nodules. Planta 115, 59–72.
Tjepkema J D, Ormerod W and Torrey J G 1980 Vesicle formation and acetylene reduction activity inFrankia sp. CpIl cultured in defined nutrient media. Nature London 287, 633–635.
Tjepkema J D, Ormerod W and Torrey J G 1981 Factors affecting vesicle formation and acetylene reduction (nitrogenase activity) inFrankia sp. CpIl. Can. J. Microbiol. 27, 815–823.
Torrey J G and Callaham D 1982 Structural features of the vesicle ofFrankia sp. CpIl in culture. Can. J. Microbiol. 28, 749–757.
Torrey J G, Tjepkema J D, Turner G L, Bergersen F J and Gibson A H 1981 Dinitrogen fixation by cultures ofFrankia sp. CpIl by15N2 incorporation. Plant Physiol. 68, 983–984.
Trinci A P J and Righelato R C 1970 Changes in constituents and ultrastructure of hyphal compartments during autolysis of glucose-starvedPenicillicem chrysogenum. J. Gen. Microbiol. 60, 239–249.
Tubb R S 1976 Regulation of nitrogen fixation inRhizobium sp. Applied Environ. Microbiol. 32, 483–488.
van Straten J, Akkermans A D L and Roelofsen W 1977 Nitrogenase activity of endophyte suspensions derived from root nodules ofAlnus, Hippophaë, Shepherdia andMyrica spp. Nature London 266, 257–258.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murry, M.A., Fontaine, M.S. & Torrey, J.G. Growth kinetics and nitrogenase induction inFrankia sp. HFPArI 3 grown in batch culture. Plant Soil 78, 61–78 (1984). https://doi.org/10.1007/BF02277840
Issue Date:
DOI: https://doi.org/10.1007/BF02277840