Abstract
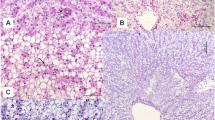
Cytosolic glutathione S-transferase (GSH transferase) activity towards 1-chloro-2,4-dinitrobenzene (CDNB) was elevated approximately three to four-fold in intestine and liver of mummichog (Fundulus heteroclitus) collected from a creosote-contaminated site in the Elizabeth River, Virginia. Intestinal GSH transferase activity at the most heavily contaminated site, at a moderately contaminated site and at a relatively clean site averaged 3.64, 2.83 and 1.11µmoles/min/mg respectively, while values for liver at these sites averaged 2.84, 1.75 and 0.93µmoles/min/mg. In addition, densitometric tracings of sodium dodecylsulfate-polyacrylamide gels of intestine and liver cytosol revealed a similar trend in the staining intensity of a 25.8 kD protein band, which lies within the molecular weight range of GSH transferase subunits. Activity in putative preneoplastic and neoplastic hepatic lesions of fish collected from the creosote-contaminated site was not significantly different from that of adjacent normal tissue. In the laboratory, dietary betanaphthoflavone (ßNF) treatment resulted in a three-fold increase in intestinal GSH transferase. Hepatic GSH transferase activity in the same fish was not affected by dietary ßNF although hepatic monooxygenase activity, measured as ethoxyresorufin O-deethylase (EROD), was. The results of this study indicate a response of the intestinal detoxification system to environmental contaminants and supports previous studies on the importance of intestinal metabolism of foreign compounds. Further, our results indicate the trend towards elevated GSH transferase in liver of feral fish could not be attributed to a cancerous disease state in these fish but indicates chemical induction in this organ as well.
Similar content being viewed by others
Abbreviations
- GSH:
-
transferase, glutathione S-transferase
- CDNB:
-
1-chloro-2, 4-dinitrobenzene
- ßNF:
-
betanaphthoflavone
- SDS-PAGE:
-
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- EROD:
-
ethoxyresorufin O-deethylase
- PAH:
-
polycyclic aromatic hydrocarbon(s)
References cited
Andersson, T., Pesonen, M. and Johansson, C. 1985. Differential induction of cytochrome P-450-dependent monooxygenase, epoxide hydrolase, glutathione transferase and UDP-glucuronosyl transferase activities in the liver of the rainbow trout by ß-naphthoflavone or Clophen A50. Biochem. Pharmacol. 34: 3309–3314.
Ankley, G. T, Blazer, V.S., Reinert, R.E. and Agosin, M. 1986. Effects of Arochlor 1254 on cytochrome P-450-dependent monooxygenase, glutathione S-transferase, and UDP-glucuronosyltransferase activities in channel catfish liver. Aquat. Toxicol. 9: 91–103.
Balk, L., Maner, S., Meijer, J., Bergstrand, A. and DePierre, J.W. 1985. Preparation and characterization of subcellular fractions from the intestinal mucosa of the Northern pike (Esox lucius), with special emphasis on enzymes involved in xenobiotic metabolism. Biochem. Biophys. Acta 838: 277–289.
Balk, L., Meijer, J, Seidegard, J., Morgenstein, R. and DePierre, J.W. 1980. Initial characterization of drug metabolizing enzymes in the liver of Northern pike (Esox lucius). Drug Metab. Dispos. 8: 98–103.
Barron, M.G., Schultz, I.R. and Hayton, W.L. 1989. Presystemic branchial metabolism limits Di-2-ethylhexyl phthalate accumulation in fish. Tox. Appl. Pharmacol. 98: 49–57.
Bieri, R.H., Hein, C., Huggett, R.J., Shou, P., Slone, H., Smith, C. and Su, C.-W. 1986. Polycyclic aromatic hydrocarbons in surface sediments from the Elizabeth River subestuary. Int. J. Environ. Anal. Chem. 26: 97–113.
Bradford, M.M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal. Biochem. 72: 248–254.
Buchmann, A., Kuhlmann, W., Schwarz, M., Kunz, W., Wolf, C.R., Moll, E., Friedberg, T. and Oesch, F. 1985. Regulation and expression of four cytochrome P-450 isoenzymes, NADPH-cytochrome P-450 reductase, the glutathione transferases B and C and microsomal epoxide hydrolase in preneoplastic and neoplastic lesions in rat liver. Carcinogenesis 6: 513–521.
Chatterjee, S. and Bhattacharya, S. 1984. Detoxication of industrial pollutants by the glutathione-S-transferase system in the liver ofAnabas testudineus (Bloch). Toxicol. Lett. 22: 187–198.
Clifton, G. and Kaplowitz, N. 1978. Effect of dietary phenobarbital, 3,4-benzo[a]pyrene and 3-methylcholanthrene on hepatic, intestinal and renal glutathione S-transferase activities in the rat. Biochem. Pharmacol. 27: 1284–1287.
Collier, T.K. and Varanasi, U. 1991. Hepatic activities of xenobiotic metabolizing enzymes and biliary levels of xenobiotics in English sole (Parophrys vetulus) exposed to environmental contaminants. Arch. Environ. Contam. Toxicol. 20: 462–473.
Connolly, J.P. and Pedersen, C.J. 1988. A thermodynamic-based evaluation of organic chemical accumulation in aquatic organisms. Environ. Sci. Technol. 22: 99–103.
Ding, V.D.-H. and Pickett, C.B. 1985. Transcriptional regulation of rat liver glutathione S-transferase genes by phenobarbital and 3-methylcholanthrene. Arch. Biochem. Biophys. 240: 553–559.
El Mouelhi, M., Didolkar, M.S., Elias, E.G., Guengerich, F.P. and Kaufman, C. 1987. Hepatic drug-metabolizing enzymes in primary and secondary tumors of human liver. Cancer Res. 47: 400–466.
Foureman, G.L. 1989. Enzymes involved in metabolism of PAH by fishes and other aquatic animals: Hydrolysis and conjugation enzymes (or Phase II enzymes).In Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment. pp. 854–865. Edited by U. Varanasi. CRC Press, Boca Raton.
George, S.G. and Buchanan, G. 1990. Isolation, properties and induction of plaice liver cytosolic glutathione-S-transferases. Fish Physiol. Biochem. 6: 437–449.
Goksøyr, A., Andersson, T., Hansson, T., Klungsoyr, J., Zhang, Y. and Förlin, L. 1987. Species characteristics of the hepatic xenobiotic and steroid biotransformation systems of two teleost fish, Atlantic cod (Gadus morhua) and rainbow trout (Salmo gairdneri). Toxicol. Appl. Pharmacol. 89: 347–360.
Habig, W.H., Pabst, M.J. and Jakoby, W.B. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130–7139.
Hammock, B.D. and Ota, K. 1983. Differential induction of cytosolic epoxide hydrolase, microsomal epoxide hydrolase and glutathione S-transferase activities. Toxicol. Appl. Pharmacol. 71: 254–265.
Hanson-Painton, O., Griffin, M.J. and Tang, J. 1983. Involvement of a cytosolic carrier protein in the microsomal metabolism of benzo[a]pyrene in rat liver. Cancer Res. 43: 4198–4206.
Hayes, M.A., Smith, I.R., Rushmore, T.H., Crane, T.L., Thorn, C., Kocal, T.E. and Ferguson, H.W. 1990. Pathogenesis of skin and liver neoplasms in white suckers from industrially polluted areas in Lake Ontario. Sci. Tot. Environ. 94: 105–123.
James, M.O., Bowen, E.R., Dansette, P.M. and Bend, J.R. 1979. Epoxide hydrase and glutathione S-transferase activities with selected alkene and arene oxides in several marine species. Chem.-Biol. Interact. 25: 321–344.
James, M.O., Heard, C.S. and Hawkins, W.E. 1988. Effect of 3-methylcholanthrene on monooxygenase, epoxide hydrolase, and glutathione S-transferase activities in small estuarine and freshwater fish. Aquat. Toxicol. 12: 1–15.
Kirby, G.M., Stalker, M., Metcalfe, C., Kocal, T., Ferguson, H. and Hayes, M.A. 1990. Expression of immunoreactive glutathione S-transferases in hepatic neoplasms induced by aflatoxin B1 or 1,2-dimethylbenzanthracene in rainbow trout (Oncorhynchus mykiss). Carcinogenesis 11: 2255–2257.
Kitahara, A., Satoh, K., Nishimura, K., Ishikawa, T., Ruike, K., Sato, K., Tsuda, H. and Ito, N. 1984. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 44: 2698–2703.
Klotz, A.V., Stegeman, J.J. and Walsh, C. 1984. An alternative 7-ethoxyresorufin 0-deethylase assay: A continuous visible spectrophotometric method for measurement of cytochrome P-450 monooxygenase activity. Anal. Biochem. 140: 138–145.
Laemmli, U.K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature, Lond. 227: 680–685.
Lindström-Seppä, P., Koivusaari, U. and Hanninen, O. 1981. Extrahepatic xenobiotic metabolism in north-European freshwater fish. Comp. Biochem. Physiol. 69C: 259–263.
Mannervik, B. and Danielson, U.H. 1988. Glutathione transferases-structure and catalytic activity. CRC Crit. Rev. Biochem. 23: 283–337.
McCain, B.B., Malins, D.C., Krahn, M.M., Brown, D.W., Gronlund, W.D., Moore, L.K. and Chan, S.-L. 1990. Uptake of aromatic and chlorinated hydrocarbons by juvenile chinook salmon (Oncorhynchus tschawytscha) in an urban estuary. Arch. Environ. Contam. Toxicol. 19: 10–16.
Mueller, J.G., Chapman, P.J. and Pritchard, P.H. 1989. Creosote-contaminated sites: their potential for bioremediation. Environ. Sci. Technol. 23: 1197–1201.
Nimmo, I.A. 1987. The glutathione S-transferases of fish. Fish Physiol. Biochem. 3: 163–172.
Roomi, M.W., Ho, R.K., Sarma, D.S.R. and Farber, E. 1985. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 45: 564–571.
Schelin, C., Tunek, A. and Jergil, B. 1983. Covalent binding of benzo[a]pyrene to rat liver cytosolic proteins and its effect on the binding to microsomal proteins. Biochem. Pharmacol. 32: 1501–1506.
Van Veld, P.A. 1990. Absorption and metabolism of dietary xenobiotics by the intestine of fish. Rev. Aquat. Sci. 2: 185–203.
Van Veld, P.A., Patton, J.S. and Lee, R.F. 1988a. Effect of preexposure to dietary benzo[a]pyrene (BP) on the first-pass metabolism of BP by the intestine of toadfish (Opsanus tau):In vivo studies using portal vein-catheterized fish. Toxicol. Appl. Pharmacol. 92: 255–265.
Van Veld, P.A., Stegeman, J.J., Woodin, B.R., Patton, J.S. and Lee, R.F. 1988b. Induction of monooxygenase activity in the intestine of spot (Leiostomus xanthurus), a marine teleost, by dietary polycyclic aromatic hydrocarbons. Drug Metab. Dispos. 16: 659–665.
Van Veld, P.A., Vetter, R.D., Lee, R.F. and Patton, J.S. 1987. Dietary fat inhibits the intestinal metabolism of the carcinogen benzo[a]pyrene in fish. J. Lipid Res. 28: 810–817.
Van Veld, P.A., Westbrook, D.J., Woodin, B.R., Hale, R.C., Huggett, R.J., Smith, C.L. and Stegeman, J.J. 1990. Induced cytochrome P-450 in intestine and liver of spot (Leiostomus xanthurus) from a polycyclic aromatic hydrocarbon contaminated environment. Aquat. Toxicol. 17: 119–132.
Vogelbein, W.K., Fournie, J.W., Van Veld, P.A. and Huggett, R.J. 1990. Hepatic neoplasms in the mummichog (Fundulus heteroclitus) from a creosote-contaminated site. Cancer Res. 50: 5978–5986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Veld, P.A., Ko, U., Vogelbein, W.K. et al. Glutathione S-transferase in intestine, liver and hepatic lesions of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Fish Physiol Biochem 9, 369–376 (1991). https://doi.org/10.1007/BF02265157
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02265157