Abstract
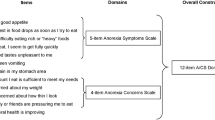
A brief visual analogue instrument was developed and tested in the context of a multicentre randomized double-blinded four-dose trial of megestrol acetate for the treatment of AIDS-related anorexia/cachexia. This nine-item instrument, the Bristol-Myers Anorexia/Cachexia Recovery Instrument (BACRI), was administered every 4 weeks after initiation of study drug (placebovs 100 mg, 400 mg or 800 mg of drug). The purpose of the instrument was to quantify patient perception of benefit in areas such as decreased concern over weight, decreased concern over appearance, increased pleasure in eating and increase in global perception of quality of life. Post-trial psychometric evaluation of the instrument strongly supported the use of a seven-item index of subjective recovery from symptoms of anorexia/cachexia (BACRI-7) and a single criterion item depicting patient perception of benefit (BACRI-1). The BACRI-7 and BACRI-1 scales showed significant improvement over 12 weeks in patients who received higher dose active drug (400 and 800 mg) compared with the placebo and 100 mg doses. Further differentiation of 400vs 800 mg arms was seen in the BACRI-7 results, consistent with dose-response improvements in weight and lean body mass changes. Quadratic trends over time in lean body mass change and provider-rated appetite grade suggested peak therapeutic effect at 8 weeks for these endpoints, whereas the absence of these trends in overall weight and patient-reported BACRI scores suggested that these benefits are more persistent. Although subjective (patient-reported) benefit is strongly associated with objective indicators of improvement, there remains the possibility that there is some added, independent benefit of megestrol acetate to subjective well-being.
Similar content being viewed by others
References
Tchekmedyian NS, Tait N, Moody M, Aisner J. High-dose megestrol acetate: A possible treatment for cachexia.JAMA 1987;257: 1195–1198.
Morgan LR. Megestrol acetate v tamoxifen in advanced breast cancer in postmenopausal patients.Semin Oncol 1985;12 (Suppl 1): 43–47.
Haller DG, Glick JH. Progestational agents in advanced breast cancer: An overview.Semin Oncol 1986;13: 2–8.
Bonomi P, Pessis D, Bunting N et al. Megestrol acetate used as primary hormonal therapy in stage D prostatic cancer.Semin Oncol 1985;12 (suppl 1): 36–39.
Tchekmedyian NS, Hickman M, Siau J, et al. Megestrol acetate in cancer anorexia and weight loss.Cancer 1992;69: 1268–1274.
Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia.J Natl Cancer Inst 1990;82: 1127–1132.
Bruera E, Macmillan K, Kuehn N, et al. A controlled trial of megestrol acetate on appetite, caloric intake nutritional status and other symptoms in patients with advanced cancer.Cancer 1990;66: 1279–1282.
Loprinzi CL, Michalak JC, Schaid DJ, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia.J Clin Oncol 1993;11: 762–767.
Von Roenn JH, Murphy RL, Weber KM et al. Megestrol acetate for the treatment of cachexia associated with human immunodeficiency virus (HIV) infection.Ann Intern Med 1988;109: 840–841.
Von Roenn JH, Armstrong D, Dickmeyer MS, et al. Megestrol acetate for the treatment of cachexia associated with human immunodeficiency virus (HIV) infection.Ann Intern Med 1994;121: 393–399.
Flynn N, Enders S, Oster M, Cone L, Hooten T. Megestrol acetate 800 mg/day vs. placebo for treatment of weight loss and anorexia in AIDS patients.Proc VIII Int Conf AIDS, Amsterdam, The Netherlands, 1992: B205 (abstract).
Cella DF. Quality of life: Concepts and definition.J Pain Symptom Management 1994;9: 186–192.
Cella DF. Quality of life: The concept.J Palliative Care 1992;8: 8–13.
Tchekmedyian NS, Hickman M, Zahyna D, Cella D. Effects of megestrol acetate on cancer patients: Impact on palliative care and quality of life. In Hartenstein R, Tchekmedyian NS:Hormones in cancer cachexia: Megestrol acetate. Munich: W. Zuchschwerdt, 1991; 59–61.
Cella DF, Bonomi AE, Leslie WT, Von Roenn J, Tchekmedyian NS. Quality of life and nutritional well-being: Measurement and relationship.Oncology 1993;7: 105–111.
Stewart AL, Hays RD, Ware JEJr. The MOS short form general health survey: Reliability and validity in a patient population.Med Care 1988;26: 724–735.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality of life instrument for use in international clinical trials in oncology.J Natl Cancer Inst 1993;85: 365–376.
Wilkinson L.SYSTAT: The System for Statistics. Evanston, IL: SYSTAT, Inc. 1990.
Robson DS. A simple method for constructing orthogonal polynomials when the independent variable is unequally spaced.Biometrics 1959;15: 187–191.
Osoba D, Murray N, Gelmon K, et al. Quality of life, appetite and weight change in patients receiving dose-intensive, weekly chemotherapy.Oncology 1994;8: 61–65.
Hays WL.Statistics (4th edn). Fort Worth, TX: Holt, Rinehart and Winston, 1988.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cella, D.F., VonRoenn, J., Lloyd, S. et al. The Bristol-Myers Anorexia/Cachexia Recovery Instrument (BACRI): a brief assessment of patients' subjective response to treatment for anorexia/cachexia. Qual Life Res 4, 221–231 (1995). https://doi.org/10.1007/BF02260861
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02260861