Summary
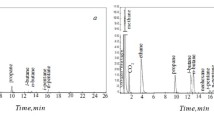
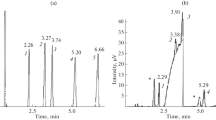
Two close-boiling materials, diethyl ether (DEE) and dichloromethane (DCM), were separated to investigate the effects of the pure components and the mixture on elution in preparative gas-liquid chromatography.
Nitrogen was used as the carrier gas, and the chromatographic column (1 cm I.D. and 0.75m length) was packed with Chromosorb A with different quantities of dinonyl phthalate stationary phase and particle sizes. Below ca. 7% (by wt.) of feed concentration, the experimental elution curves of pure DEE and DCM were almost the same as those of the mixture, and the theoretical plate model can be used successfully to predict the elution curves.
Similar content being viewed by others
Abbreviations
- a:
-
constant equal to 1/(Vm+Kvs)
- K:
-
partition coefficient
- N:
-
number of theoretical plates
- tR :
-
distance from injection to peak maximum (cm)
- V:
-
volume of the gas phase (cm3)
- vm :
-
void volume in a plate (cm3)
- vs :
-
volume of stationary phase in a plate (cm3)
- w:
-
length of the base line cut by the two tangents of the peak (cm)
- Xn :
-
concentration of solute in the stationary phase (mol/L)
- Y oi :
-
initial concentration of solute in the gas phase on the ith plate (mol/L)
- Yn :
-
concentration of solute in the gas phase (mol/L)
- Yo :
-
feed concentration (mol/L)
References
W. H. Husband, P. E. Barker, K. D. Kini, Trans. Instn. Chem. Engrs.,42, T387 (1964).
D. H. James, C. S. G. Phillips J. Chem. Soc., 1600 (1953).
K. H. Row, W. K. Lee, “Separation by Gas — Liquid Chromatography,” Cheremisinoff ed., Handbook of Heat and Mass Transfer, Vol. 3, Gulf Publisher, Chapter 22, 1988, p. 883.
M. A. Alkharasani, B. J. McCoy, Chem. Eng. J.,23, 81 (1982).
K. H. Row, W. K. Lee, J. of Chem. Eng. of Japan,19, 173 (1986).
K. H. Row, W. K. Lee Korean J. of Chem. Eng.,3, 7 (1986).
C. Bosanquet, G. D. Morgan, in “Vapour” Phase Chromatography”. Desty D. H. Ed. Butterworths, London, 1957; p. 35.
H. Y. Ha, K. H. Row W. K. Lee, Sep. Sci. and Technol.,22, 141 (1987).
J. C. Giddings, “Dynamics of Chromatography”, Part I, Dekker, New York, 1965; p. 20.
I. Moon, K. H. Row, W. K. Lee, Korean J. of Chem. Eng.,2, 155 (1985).
H. M. MacNair, E. J. Bonelli, “Basic Gas Chromatography,” Varian Instrument Division, Berkeley, California, 1969; p. 49.
C. Y. Wen, L. T. Fan, “Models for Flow Systems and Chemical Reactors,” Dekker, New York; 1975, p. 169.
A. T. James, A. J. P. Bonelli, Analyst.77, 915 (1952).
K. H. Row, “Combined Continuous and Preparative Separation of Close-Boiling Components in a Gas — Liquid Chromatography,” Ph. D. dissertation, KAIST, Seoul (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Row, K.H. Comparison of the elution curves of pure components and their mixture and prediction of elution curves by a theoretical plate model in preparative gas-liquid chromatography. Chromatographia 25, 961–964 (1988). https://doi.org/10.1007/BF02259412
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02259412