Abstract
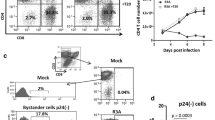
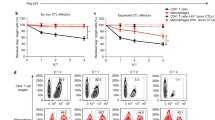
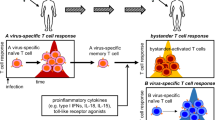
The induction of apoptosis in T cells by bystander cells has been repeatedly implicated as a mechanism contributing to the T cell depletion seen in HIV infection. It has been shown that apoptosis could be induced in T cells from asymptomatic HIV-infected individuals in a Fas-independent, TNF-related apoptosis-inducing ligand (TRAIL)-dependent manner if the cells were pretreated with anti-CD3. It has also been shown that T cells from HIV-infected patients were even more sensitive to TRAIL induction of apoptosis than they were to Fas induction. Recently, it has been reported that in an HIV-1 SCID-Hu model, the vast majority of the T cell apoptosis is not associated with p24 and is therefore produced by bystander effects. Furthermore, few apoptotic cells were associated with neighboring cells which were positive for either Fas ligand or TNF. However, most of the apoptotic cells were associated with TRAIL-positive cells. The nature of these TRAIL-positive cells was undetermined. Here, we report that HIV infection of primary human macrophages switches on abundant TRAIL production both at the RNA and protein levels. Furthermore, more macrophages produce TRAIL than are infected by HIV, indicating that a bystander mechanism may, at least in part, upregulate TRAIL. Exogenously supplied HIV-1 Tat protein upregulates TRAIL production by primary human macrophages to an extent indistinguishable from infection. The results suggest a model in which HIV-1-infected cells produce extracellular Tat protein, which in turn upregulates TRAIL in macrophages which then can induce apoptosis in bystander T cells.
Similar content being viewed by others
References
Albini A, Benelli R, Giunciuglio D, Cai T, Mariani G, Ferrini S, Noonan DM. Identification of a novel domain of HIV Tat involved in monocyte chemotaxis. J Biol Chem 273:15895–15900;1998.
Ameisen JC, Capron A. Cell dysfunction and depletion in AIDS: The programmed cell death hypothesis. Immunol Today 12:102–105;1991.
Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol 70:199–206;1996.
Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly RP, Finkel TH. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med 176:1099–1106;1992.
Bartz SR, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol 73:1956–1963;1999.
Brian J. Inophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol 159:3823–3837;1997.
Cottrez F, Manca F, Dalgleish AG, Arenzana-Seisdedos F, Capron A, Groux H. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. J Clin Invest 99:257–266;1997.
Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186:1165–1170;1997.
Dockrell D, Andrew H, Badley D, Villacian JS, Heppelmann CJ, Algeciras A, Ziesmer S, Yagita H, Lynch DH, Roche PC, Leibson PJ, Paya CV. The expression of Fas Ligand by macrophages and its upregulation by human immunodeficiency virus infection. J Clin Invest 101:2394–2405;1998.
Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84–86;1990.
Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med 1:129–134;1995.
Gibellini D, Zauli G, Re MC, Milani D, Furlini G, Caramelli E, Capitani S, Placa ML. Recombinant human immunodeficiency virus type-1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF-beta 1 mRNA expression and protein synthesis in peripheral blood monocytes. Br J Haematol 88:261–267;1994.
Gougeon ML, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retroviruses 9:553–563;1993.
Groux H, Torpier GK, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med 175:331–340;1992.
Herbein G, Van Lint C, Lovett JL, Verdin E. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J Virol 72:660–670;1998.
Huang L, Bosch I, Hofmann W, Sodroski J, Pardee AB. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol 72:8952–8960;1998.
Jaworowski A, Crowe SM. Does HIV cause depletion of CD4+ T cells in vivo by the induction of apoptosis? Immunol Cell Biol 77:90–98;1999.
Jeremias I, Herr I, Boehler T, Debatin KM. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol 28:143–152;1998.
Katsikis PD, Garcia-Ojeda ME, Torres-Roca JF, Tijoe IM, Smith CA, Herzenberg LA, Herzenberg LA. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med 186:1365–1372;1997.
Kolesnitchenko V, King L, Riva A, Tani Y, Korsmeyer SJ, Cohen DI. A major human immunodeficiency virus type 1-initiated killing pathway distinct from apoptosis. J Virol 71:9753–9763;1997.
Lafrenie RM, Wahl LM, Epstein JS, Hewlett IK, Yamada KM, Dhawan S. HIV-1-Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. A mechanism of HIV pathogenesis. J Immunol 156:1638–1645;1996.
Lafrenie RM, Wahl LM, Epstein JS, Hewlett IK, yamada KM, Dhawan S. HIV-1-Tat protein promotes chemotaxis and invasive behavior by monocytes. J Immunol 157:974–977;1996.
Lafrenie RM, Wahl LM, Epstein JS, Yamada KM, Dhawan S. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J Immunol 159:4077–4083;1997.
Lewis DE, Ng-Tang DS, Adu-Oppong A, Schober W, Rodgers JR. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol 153:412–420;1994.
Li CJ, Friedman DF, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429–431;1995.
Li CJ, Ueda Y, Shi B, Borodyansky L, Huang L, Li YZ, Pardee AB. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci USA 94:13832–13837;1997.
Marsters SA, Pitti RM, Donahue CJ, Ruppert S, Bauer KD, Ashkenazi A. Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr Biol 6:750–752;1996.
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 7:1003–1006;1997.
Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RPM, Miedema F. Programmed death of T cells in HIV-1 infection. Science 257:217–219;1992.
Mongkolsapaya J, Cowper AE, Xu XN, Morris G, McMichael AJ, Bell JI, Screaton GR. Lymphocyte inhibitor of TRAIL (TNF-related apoptosis-inducing ligand): A new receptor protecting lymphocytes from the death ligand TRAIL. J Immunol 160:3–6;1998.
Mosier D, Sieburg H. Macrophage-tropic HIV: Critical for AIDS pathogenesis? Immunol Today 15:332–339;1994.
Mosier DE, Gulizia RJ, MacIsaac PD, Torbett BE, Levy JA. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260:689–692;1993.
Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol 154:5555–5566;1995.
Nagata S. Apoptosis by death factor. Cell 88:355–365;1997.
Neuveut C, Jeang K-T. Recombinant human immunodeficiency virus type 1 genomes withtat unconstrained by overlapping reading frames reveal residues in Tat important for replication in tissue culture J Virol 70:5572–5581;1996.
Ott M, Lovett JL, Mueller L, Verdin E. Super-induction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-kappaB factors. J Immunol 160:2872–2880;1998.
Oyaizu N, Adachi Y, Hashimoto F, McCloskey TW, Hosaka N, Kayagaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: A possible mechanism of bystander cell death in HIV infection. J Immunol 158:2456–2463;1997.
Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood 82:3392–3400;1993.
Pan G, Ni J, Wei YF, Yu GI, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818;1997.
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science 276:111–113;1997.
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271:12687–12690;1996.
Sarin A, Clerici M, Blatt SP, Hendrix CW, Shearer GM, Henkart PA. Inhibition of activation-induced programmed cell death and restoration of defective immune responses of HIV+ donors by cysteine protease inhibitors. J Immunol 153:862–872;1994.
Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol 7:693–696;1997.
Sheridan JP, Marsters SA, Pitti PM, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818–821;1997.
Sieg S, Smith D, Yildirim Z, Kaplan D. Fas ligand deficiency in HIV disease. Proc Natl Acad Sci USA 94:5860–5865;1997.
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: A novel apoptosis-mediating receptor for TRAIL. EMBO J 16:5386–5397;1997.
Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500;1995.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682;1995.
Wu MX, Schlossman SF. Decreased ability of HIV-1 tat protein-treated accessory cells to organize cellular clusters is associated with partial activation of T cells. Proc Natl Acad Sci USA 94:13832–13837;1997.
Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA 97:11466–11471;2000.
Zauli G, Gibellini D. The human immunodeficiency virus type-1 (HIV-1) Tat protein and Bcl-2 gene expression. Leuk Lymphoma 23:551–560;1996.
Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M, Capitani L. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 86:3823–3834;1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, M., Li, X., Pang, X. et al. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: Upregulation of TRAIL in primary human macrophages by HIV-1 tat. J Biomed Sci 8, 290–296 (2001). https://doi.org/10.1007/BF02256603
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02256603