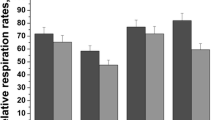
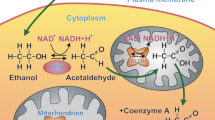
Abstract
Alcohol-induced oxidative stress is linked to the metabolism of ethanol. Three metabolic pathways of ethanol have been described in the human body so far. They involve the following enzymes: alcohol dehydrogenase, microsomal ethanol oxidation system (MEOS) and catalase. Each of these pathways could produce free radicals which affect the antioxidant system. Ethanol per se, hyperlactacidemia and elevated NADH increase xanthine oxidase activity, which results in the production of superoxide. Lipid peroxidation and superoxide production correlate with the amount of cytochrome P450 2E1. MEOS aggravates the oxidative stress directly as well as indirectly by impairing the defense systems. Hydroxyethyl radicals are probably involved in the alkylation of hepatic proteins. Nitric oxide (NO) is one of the key factors contributing to the vessel wall homeostasis, an important mediator of the vascular tone and neuronal transduction, and has cytotoxic effects. Stable metabolites — nitrites and nitrates — were increased in alcoholics (34.3±2.6 vs. 22.7±1.2 µmol/l, p<0.001). High NO concentration could be discussed for its excitotoxicity and may be linked to cytotoxicity in neurons, glia and myelin. Formation of NO has been linked to an increased preference for and tolerance to alcohol in recent studies. Increased NO biosynthesis also via inducible NO synthase (NOS, chronic stimulation) may contribute to platelet and endothelial dysfunctions. Comparison of chronically ethanol-fed rats and controls demonstrates that exposure to ethanol causes a decrease in NADPH diaphorase activity (neuronal NOS) in neurons and fibers of the cerebellar cortex and superior colliculus (stratum griseum superficiale and intermedium) in rats. These changes in the highly organized structure contribute to the motor disturbances, which are associated with alcohol abuse. Antiphospholipid antibodies (APA) in alcoholic patients seem to reflect membrane lesions, impairment of immunological reactivity, liver disease progression, and they correlate significantly with the disease severity. The low-density lipoprotein (LDL) oxidation is supposed to be one of the most important pathogenic mechanisms of atherogenesis, and antibodies against oxidized LDL (oxLDL) are some kind of epiphenomenon of this process. We studied IgG oxLDL and four APA (anticardiolipin, antiphosphatidylserine, antiphosphatidylethanolamine and antiphosphatidylcholine antibodies). The IgG oxLDL (406.4±52.5 vs. 499.9±52.5 mU/ml) was not affected in alcoholic patients, but oxLDL was higher (71.6±4.1 vs. 44.2±2.7 µmol/l, p<0.001). The prevalence of studied APA in alcoholics with mildly affected liver function was higher than in controls, but not significantly. On the contrary, changes of autoantibodies to IgG oxLDL revealed a wide range of IgG oxLDL titers in a healthy population. These parameters do not appear to be very promising for the evaluation of the risk of atherosclerosis. Free radicals increase the oxidative modification of LDL. This is one of the most important mechanisms, which increases cardiovascular risk in chronic alcoholic patients. Important enzymatic antioxidant systems — superoxide dismutase and glutathione peroxidase — are decreased in alcoholics. We did not find any changes of serum retinol and tocopherol concentrations in alcoholics, and blood and plasma selenium and copper levels were unchanged as well. Only the zinc concentration was decreased in plasma. It could be related to the impairment of the immune system in alcoholics. Measurement of these parameters in blood compartments does not seem to indicate a possible organ, e.g. liver deficiency.
Similar content being viewed by others
References
Adams ML, Cicero TJ. Alcohol intoxication and withdrawal: The role of nitric oxide. Alcohol 16:153–158;1998.
Ahmed S, Leo MA, Lieber CS. Interactions between alcohol and beta-carotene in patients with alcoholic liver disease. Am J Clin Nutr 60:430–436;1994.
Alarcon-Segovia D, Delezé M, Oria CV. Antiphospholipid antibodies and the antiphospholipid syndrome in systemic lupus erythematosus: A prospective analysis of 500 consecutive patients. Medicine 68:353–374;1989.
Anggard E. Nitric oxide: Mediator, murder, and medicine. Lancet 343:1199–1206;1994.
Asherson RA. Antiphospholipid antibodies and syndromes. In: Lahita RG, ed. Systemic Lupus erythematosus. New York, Churchill Livingstone, 587–635;1992.
Berdeaux A. Nitric oxide: An ubiquitous messenger. Fundam Clin Pharmacol 7:401–411;1993.
Berger MM. Rôle des oglio-éléments et des vitamines en nutrition péri-opérative. Ann Fr Anesth Réanim 14:82–84;1995.
Berliner J, Heinecke JW. The role of oxidized lipoproteins in atherosclerosis. Free Radic Biol Med 20:707–727;1996.
Bird G, Mills P, Smith D, Runcie J. Antibodies to phospholipid in alcoholic liver diseases. Br Med J 309:1161;1994.
Biron C, Andreani H, Blanc P, Ramos J, Ducos J, Guigue N, Michel H, Larrez D, Schved JF: Prevalence of antiphospholipid antibodies in patients with chronic liver disease related to alcohol or hepatitis C virus: Correlation with liver injury. J Lab Clin Med 131:243–250;1998.
Bjorneboe GE, Johnsen J, Bjorneboe A, Bache-Wiig JE, Morland J, Drevon CA. Diminished serum concentration of vitamin E in alcoholics. Ann Nutr Metab 32:56–61;1988.
Bjorneboe GA, Johnsen J, Bjorneboe A, Morland J, Drevon CA. Effect of heavy alcohol consumption on serum concentrations of fat-soluble vitamins and selenium. Alcohol Alcohol Suppl 22:533–537;1987.
Bjorneboe GE, Johnsen J, Bjorneboe A, Marklund SL, Skylv N, Hoiseth A, Bache-Wiig JE, Morland J, Drevon CA. Some aspects of antioxidant status in blood from alcoholics. Alcohol Clin Exp Res 12:806–810;1988.
Borovanský J. Detection of metals in tissues, cells and subcellular organelles. Sbor Lék 98:77–97;1997.
Brody T. Vitamin A. In: Brody T, ed. Nutritional Biochemistry. San Diego, Academic Press, 400–409;1994.
Brody T. Vitamin E. In: Brody T, ed. Nutritional Biochemistry. San Diego, Academic Press, 459–463;1994.
Chedid A, Chadalawada KR, Morgan TR, Moritz TE, Mendenhall CL, Hammond JB, Emblad PW, Cifuentes DC, Kwak JW, Gilman-Sachs A. Phospholipid antibodies in alcoholic liver disease. Hepatology 20:1465–1471;1994.
Chvapil M. New aspects in the biological role of zinc, a stabilizer of macromolecules and biological membranes. Life Sci 13:1041–1049;1973.
Cousins RJ. Zinc. In: Ziegler EE, Filer IJ Jr, eds. Present Knowledge in Nutrition, ed 7. Washington, ILSI Press, 293–306;1996.
Crkovská J, Štípek S. Factors influencing assay for nitrite and nitrate in serum with the use of nitrate reductase and Griess reagent. Klin Biochem Metab 27:82–87;1998.
Darley-Usmar V, Halliwel B. Blood radicals — Reactive nitrogen species, reactive oxygen species, transition metall ions, and the vascular system. Pharmacol Res 13:649–662;1996.
Esterbauer H, Dieber-Rotheneder M, Waeg G, Striegl G, Jurgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol 3:77–92;1990.
Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol 127:31–64;1996.
Esterbauer H. Estimation of peroxide damage: A critical review. Pathol Biol 44:25–28;1996.
Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes: Characterization, purification, molecular cloning, and function. Hypertension 23:1121–1131;1994.
Fraker PJ, Jardieu P, Cook J. Zinc deficiency and immune function. Arch Dermatol 123:1699–1701;1987.
Gervais A, Czernichow B, Grunebaum L, Wiesel ML, Auperin A, Rivalland D, Gabanyi J, Goldstein L, Cazenave JP, Doffoel M. Prevalence of serum anticardiolipin antibodies in alcoholic cirrhosis. Gastroenterol Clin Biol 20:736–742;1996.
Gey FK, Puska P, Jordan P, Moser UK. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-culture epidemiology. Am J Clin Nutr 53:326S-334S;1991.
Griffith RL, Virella GT, Stevenson HC, Lopes-Virella MF. Low-density lipoprotein metabolism by human macrophages activated with low-density lipoprotein immune complexes. A possible mechanism of foam cell formation. J Exp Med 168:1041–1059;1988.
Halliwell B. Drug antioxidant effects: A basis for drug selection? Drugs 42:569–605;1991.
Harris N. Antiphospholipid antibodies. Br J Haematol 74:1–9;1996.
Hoening M, Kesabiec AM. Sample preparation steps for analysis by atomic spectroscopy methods: Present status. Spectrochim Acta Part B 51:1297–1307;1996.
Horkko S, Miller E, Dudl E, Reaven P, Curitss LK, Zvaifler NJ, Terkeltaub R, Pierangeli SS, Branch DW, Palinski W, Witztum JL. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids: Recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J Clin Invest 98:815–825;1996.
Hunt NC, Goldin R. Nitric oxide production by monocytes in alcoholic liver disease. J Hepatol 14:146–150;1992.
Janebová M, Zima T. Methods for determination of vitamins A and E — Our simple HPLC assay. Sbor Lék 98:195–208;1997.
Khanna JM, Morato GS, Shah G, Chau A, Kalant H. Inhibition of nitric oxide synthesis impairs rapid tolerance to ethanol. Brain Res Bull 32:43–47;1993.
Lancaster FE. Alcohol and the brain: What's NO got to do with it? Metab Brain Dis 10:125–133;1995.
Lapin A, Temml CH, Wonish W. Antibodies against oxidized LDL (oLAb) in Viennese working population. Sborník FONS, Symposium of Clinical Biochemistry, Luhaćovice, 13;1996.
Laskin CA, Vidins E, Blendis LM, Soloninka CA. Autoantibodies in alcoholic liver disease. Am J Med 89:129–133;1990.
Lecomte E, Herbeth B, Pirollet P, Chancerelle Y, Arnaud J, Musse N, Paille F, Siest G, Artur Y. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am J Clin Nutr 60:255–261;1994.
Lecomte E, Grolier P, Herbeth B, Pirollet P, Musse N, Paille F, Braesco V, Siest G, Artur Y. The relation of alcohol consumption to serum carotenoid and retinol levels: Effects of withdrawal. Int J Vitam Nutr Res 64:170–175;1994.
Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidized LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not by vitamin E. Circulation 91:1525–1532;1995.
Levander OA, Burk RF. Selenium. In: Ziegler EE, Filer IJ Jr, eds. Present Knowledge in Nutrition, ed 7. Washington, ILSI Press, 320–328;1996.
Liew FY. The role of nitric oxide in parasitic diseases. Ann Trop Med Parasitol 87:637–642;1993.
Lin RC, Dai J, Lumeng L, Zhang MY. Serum low density lipoprotein of alcoholic patients ischemically modified in vivo and induced apolipoprotein E synthesis by macrophages. J Clin Invest 95:1979–1986;1995.
Linder MC. Nutrition and Metabolism of the Trace Elements. In: Linder MC, ed. Nutritional Biochemistry and Metabolism with Clinical Application, ed. 2. Englewood Cliffs, Prentice Hall, 215–276;1991.
Linder MC. Copper. In: Ziegler EE, Filer LJ Jr, eds. Present Knowledge in Nutrition, ed 7. Washington, ILSI Press, 307–319;1996.
London GM, Druecke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int 51:1678–1695;1997.
Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: A physiologic messenger. Ann Intern Med 120:227–237;1994.
Maggie E, Finardi G, Pli M, Bollati P, Filipponi M, Stefano PL, Paolini G, Grossi A, Clot P, Albano E. Specificity of autoantibodies against oxidized LDL as an aditional marker for atherosclerosis risk. Coronary Art Dis 4:1119–1122;1993.
Mangia A, Margaglione M, Cascavilla I, Gentile R, Cappucci G, Facciorusso D, Grandone E, Di Minno G, Rizzetto M, Andriulli A. Anticardiolipin antibodies in patients with liver disease. Am J Gastroenterol 94:2983–2987;1999.
McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med 26:1034–1053;1999.
Menzano E, Carlen PL. Zinc deficiency and corticosteroids in the pathogenesis of alcoholic brain dysfunction — A review. Alcohol Clin Exp Res 18:895–901;1994.
Mestek O, Suchánek M, Vodičková Z, Zemanová B, Zima T. Comparison of the suitability of various atomic spectroscopic techniques for the determination of selenium in human whole blood. J Anal Atom Spectrometry 12:85–87;1997.
Mestek O, Čcaron;urodová E, Koplík R, Zima T. Přímé stanovení mědi a zinku v pliné lidské krvi metodou ICP-MS. Chem listy 91:1059–1062;1997.
Molina JA, Bermejo F, del Ser T, Jimenez-Jimenez FJ, Herranz A, Fernandez-Calle P, Ortuno B, Villanueva C, Sainz MJ. Alcoholic cognitive deterioration and nutritional deficiencies. Acta Neurol Scand 89:384–390;1994.
Navder KP, Baroana E, Leo MA, Lieber CS. Oxidation of LDL in baboons is increased by alcohol and attenuated by polyenylphosphatidylcholine. J Lipid Res 40:983–987;1999.
Neiman J, Benthin G. Nitric oxide is not increased in alcoholic brain. Alcohol Alcohol 32:551–553;1997.
Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol 29:513–522;1994.
Olson JA. Carotenoids and vitamin A: An overview. In: Ong ASH, Packer L, eds. Lipid-Soluble antioxidants: Biochemistry and Clinical Applications. Basel, Birkhäuser, 178–192;1992.
Orlando R, Tosone G, De Fino M, Cangiano F. Changes in the levels of serum zinc in chronic hepatopathy. Minerva Med 78:1759–1763;1987.
Oswald IP, Wynn TA, Sher A, James SL. NO as an effector molecule of parasite killing. Comp Biochem Physiol Pharmacol Toxicol Endocrinol 108:11–18;1994.
Packer L. New horizons in vitamin E research — The vitamin E cycle, biochemistry and clinical applications. In: Ong ASH, Packer L, eds. Lipid-Soluble Antioxidants: Biochemistry and Clinical Applications. Basel, Birkhäuser, 1–16;1992.
Paiker JE, Raal FJ, von Arb M. Auto-antibodies against oxidized LDL as a marker for coronary artery disease in patients with familiar hypercholesterolaemia. Ann Clin Biochem 37:74–178;2000.
Parlesak A, Schafer Ch, Schutz T, Bode JCh, Bode Ch. Increased intestinal permeability and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32:742–747;2000.
Parthasarathy S, Rankin SM. Role of oxidized low density lipoprotein in atherogenesis. Prog Lipid Res 31:127–132;1992.
Persson MG, Gustafsson LE. Ethanol can inhibit nitric oxide production. Eur J Pharmacol 224:99–100;1992.
Puddey IB, Croft KD. Alcohol, stroke and coronary heart disease. Neuroepidemiology 18:292–302;1999.
Rifici VA, Stephan EM, Schneider SH, Khachdurian AK. Red wine inhibits the cell-mediated oxidation of LDL and HDL. J Am Coll Nutr 18:137–143;1999.
Ringstad J, Knutsen SF, Nilssen OR, Thomassen Y. A comparative study of serum selenium and vitamin E levels in a population of male risk drinkers and abstainers: A population-based matched-pair study. Biol Trace Elem Res 36:65–71;1993.
Ross R. The pathogenesis of atherosclerosis — An update. N Engl J Med 335:488–500;1996.
Ross R. Atherosclerosis — An inflammatory disease. New Engl J Med 340:115–126;1999.
Sanchez-Rodriguez A, Criado M, Rodriguez-Lopez AM, Esteller A, Martin de Arriba A, Lopez-Novoa JM. Increased nitric oxide synthesis and inducible nitric oxide synthase expression in patients with alcoholic and non-alcoholic liver cirrhosis. Clin Sci (Colch) 94:637–643;1998.
Schved JF. Prevalence of antiphospholipid antibodies in patients with chronic liver disease related to alcohol or hepatitis C virus: Correlation with liver injury. J Lab Clin Med 131:243–250;1998.
Sies H, Murphy ME, Di Mascio P, Stahl W. Tocopherols, carotenoids and the glutathione system. In: Ong ASH, Packer L, eds. Lipid-Soluble Antioxidants: Biochemistry and Clinical Applications. Basel, Birkhäuser, 160–165;1992.
Tanabe N, Toyoshima H, Hayashi S, Miyanishi K, Funazaki T, Obata A, Wakai S, Enoki S, Hashimoto S, Kamimura K. Effects of smoking and drinking habits and vitamin A intake on serum concentrations of beta-carotene and retinol. Nippon Eiseigaku Zasshi 47:679–687;1992.
Toyoshima H, Hayashi S, Miyanishi K, Wakai S, Enoki S, Kumagai H, Kamimura K. Effects of serum lipid concentrations and smoking and drinking habits on serum vitamin A and E levels. Nippon Eiseigaku Zasshi 44:659–666;1989.
Vaarala O, Alfthan G, Jauhiainen M. Cross-reaction between antibodies to oxidized low-density lipoprotein and to cardiolipin in systemic lupus erythematodes. Lancet 341:923–925;1994.
Versieck J, Cornelis R. Normal levels of trace elements in human blood plasma or serum. Anal Chim Acta 116:217–254;1980.
Ward RJ, Peters TJ. The antioxidant status of patients with either alcohol-induced liver damage or myopathy. Alcohol Alcohol 27:359–365;1992.
Wehr H, Milewski B, Pozniak M, Rodo M. Anti-low density lipoprotein antibodies in alcoholics without and with liver disease and in social drinkers. Alcohol Alcohol 32:43–49;1997.
Witzum JL. The oxidative hypothesis of atherosclerosis. Lancet 344:793–795;1994.
Yla-Herttuala S. Macrophages and oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Ann Med 23:561–567;1991.
Zarski JP, Arnaud J, Labadie H, Beaugrand M, Favier A, Rachail M. Serum and tissue concentrations of zinc after oral supplementation in chronic alcoholics with and without cirrhosis. Gastroenterol Clin Biol 11:856–860;1987.
Zima T, Fialová L, Mikulíková L, Matouš Malbohan I, Popov P, Nešpor K. Antibodies against phospholipids and oxidized LDL in alcoholic patients. Physiol Res 47:351–355;1998.
Zima T. Oxid dusnatý /NO/ — fyziologické a patofyziologické účinky v organismu. Remedia 7:298–307:1997.
Zima T, Fialová L, Němeček K, Mikulíková L, Tesař V, Merta M, Chábová V, Bártová V, Malbohan I, Štípek S. IgG antibody to oxidized low-density lipoprotein — Is it a marker of atherogenesis in patients with renal diseases? In: Timio M, Wizemann V, Venanzi S, eds. Cardionephrology 4. Cosenza, Editoriale Bios, 23–24;1997.
Zima T, Crkovská J, Štípek S. Spectrophotometric assay of oxidized low-density lipoprotein. Klin Biochem Metab 27:72–76;1998.
Zima T, Druga R, Štípek S. The influence of chronic moderate ethanol administration on NADPH-diaphorase /NO synthase/ activity in rats brain. Alcohol Alcohol 33:341–346;1998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zima, T., Fialová, L., Mestek, O. et al. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci 8, 59–70 (2001). https://doi.org/10.1007/BF02255972
Issue Date:
DOI: https://doi.org/10.1007/BF02255972