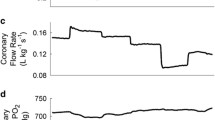
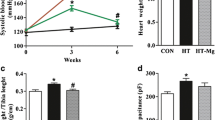
Abstract
This study quantitatively examined myocardial changes during the hypertensive process. Four groups (n=10 for each group) of adultRattus norvegicus (Wistar strain) were studied. Animals were sacrificed at 40 and 80 days of experimentation (control and experimental groups in each age). Although animals in the experimental groups received L-NAME (50 mg/kg/day) for 40 days, one group of animals was without L-NAME for the remaining 40 days. In addition, stereology was performed to determine the volume and numerical densities of myocytes (Vv and Nv, respectively), the mean volume and the total number of myocytes (Vol and N, respectively). According to those results, the blood pressure increased following L-NAME administration and remained high even at 40 days after administering L-NAME. The cardiac weight significantly increased in L-NAME animals and also in control of older animals. Moreover, Vv and the Vol increased in older animals. Notably, inhibition of the NO synthase increased Vol while decreasing Nv and N. These indices remained unchanged even after 40 days of the L-NAME intake interruption. Nv and N also decreased in older animals. Furthermore, hypertrophy and loss of myocytes were not entirely reversible after cessation of the chronic inhibition of the NO synthase in an extended follow-up.
Similar content being viewed by others
References
Anversa P, Li P, Zhang X, Ollivetti G, Capasso JM. Ischemic myocardium injury and ventricular remodeling. Cardiovasc Res 27:145–157;1993.
Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res 67:871–885;1990.
Arnal JF, El Amrani AI, Chatellier G, Ménard J, Michel JB. Cardiac weight in hypertension induced by nitric oxide synthase blockade. Hypertension 22:380–387;1993.
Baba HA, Takeda A, Schmid C, Nagano M. Early proliferate changes in hearts of hypertensive Goldblatt rats: An immunohistochemical and flow-cytometrical study. Basic Res Cardiol 91:275–282;1996.
Bing OHL. Hypothesis: Apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol 26:943–948;1994.
Borg TK, Caulfield JB. The collagen matrix of the heart. Fed Proc 40:2037–2041;1981.
Brilla CG, Maisch B, Zhou G, Weber KT. Renin-angiotensin system and myocardial collagen matrix remodeling in hypertensive heart disease: In vivo and in vitro studies on collagen matrix regulation. Clin Invest 71:35–41;1993.
Brömme HJ, Holtz J. Apoptosis in the heart: When and why? Mol Cell Biochem 163/164:261–275;1996.
Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: A brief survey. Am J Physiol 258:L148-L156;1990.
Del Monte F, O'Gara P, Poole-Wilson PA, Yacoub M, Harding SE. Cell geometry and contractile abnormalities of myocytes from failing human left ventricle. Cardiovasc Res 30:281–290;1995.
Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res 79:949–956;1996.
Gardiner SM, Kemp PA, Bennett T, Palmer RMJ, Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquaters vasoconstriction in Brattleboro rats. Eur J Pharmacol 213:449–451;1992.
Gerdes AM, Capasso JM. Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. J Mol Cell Cardiol 27:849–856;1995.
Gerdes AM, Onodera T, Wang X, Mc Cune AS. Myocyte remodeling during the progression to failure in rats with hypertension. Hypertension 28:609–614;1996.
Gerová M, Hartmannová B, Dolezel S, Jezek L. Long term inhibition of NO synthase induces cardiac hypertrophy with a decrease in adrenergic innervation. Physiol Res 45:339–344;1996.
Glennon PE, Sugden PH, Poole-Wilson PA. Cellular mechanims of cardiac hypertrophy. Br Heart J 73:496–499;1995.
Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg G, Sørensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881;1988.
Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol 271:H1215-H1228;1996.
Kajstura J, Zhang X, Reiss K, Szoke E, Li P, Lagrasta C, Cheng W, Darzynkiewicz Z, Olivetti G, Anversa P. Myocyte cellular hyperplasia and myocyte cellular hypertrophy contribute to chronic ventricular remodeling in coronary artery narrowing-induced cardiomyopathy in rats. Circ Res 74:383–400;1994.
Leonetti G, Cuspidi C. The heart and vascular changes in hypertension. J Hypertens 13(suppl 2):529–534;1995.
Levy D, Garrison RJ, Savage DD, Kannel WB, Casteli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham study. N Engl J Med 322:1561–1566;1990.
Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol 5:370–382;1960.
Mandarim-de-Lacerda CA, Pereira LMM. Stereology of the myocardium in hypertensive rats under chronic inhibition of nitric oxide synthesis. Biomed Res 8:153–160;1997.
Mandarim-de-Lacerda CA, Santos MB, Le Floch-Prigent P, Narcy F. Stereology of the myocardium in human foetuses. Early Hum Dev 48:249–259;1997.
Matfeldt T, Möbius HJ, Mall G. Orthogonal triplet probes: An efficient method for unbiased estimation of length and surface of objects with unknown orientation in space. J Microsc 139:279–289;1985.
Mayhew TM, Gundersen HJG. ‘If you assume, you can make as ass out of u and me’: A decade of the disector for stereological counting of particles in 3D space. J Anat 188:1–15;1996.
Moncada S, Higgs EA. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur J Clin Invest 21:361–374;1991.
Moreno H, Metze K, Bento AC, Antunes E, Zata R, Nucci G. Chronic nitric oxide inhibition as a model of hypertensive heart muscle disease. Basic Res Cardiol 91:248–255;1996.
Narula J, Haider N, Virmani R, Disalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran M, Dec W, Khaw BA. Apoptosis in myocytes in end stage heart failure. N Engl J Med 335:1182–1189;1996.
Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064;1992.
Nguyen ND, Buja LM. The role of ventricular wall stress in cardiac hypertrophy. Cardiovasc pathol 3:19–31;1994.
Numaguchi K, Egashira K, Takemoto M, Kadokami T, Shimokawa H, Sueishi K, Takeshita A. Chronic inhibition of nitric oxide synthesis causes coronary microvascular remodeling in rats. Hypertension 26:957–962;1995.
Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Moll Cell Cardiol 28:1463–1477;1996.
Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: Effects on the human heart. J Am Coll Cardiol 26:1068–1079;1995.
Olivetti GM, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart: Myocyte loss and reactive cellular hypertrophy. Circ Res 68:1560–1568;1991.
Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526;1987.
Pereira LMM, Mandarim-de-Lacerda CA. Morphology of the myocardium in rats under chronic inhibition of the nitric oxide synthesis. Rev Chil Anat 14:147–155;1996.
Pereira LMM, Mandarim-de-Lacerda CA. Sterology of the myocardium in hypertensive rats by the use of the nitric oxide synthase inhibition. Rev Port Cardiol 16:753–758;1997.
Pörsti I, Paakkari I. Nitric oxide based possibilities for pharmacotherapy. Ann Med 27:407–420;1995.
Rakusan K. Cardiac growth, maturatin and ageing. In: Zak R, ed. Growth of the Heart in Health and Disease. New York, Raven Press, 131–164;1984.
Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: An insight into endotoxin shock. Biochem Biophys Res Commum 173:541–547;1990.
Regan CP, Anderson PG, Bishop SP, Berecek KH. Captopril prevents vascular and fibrotic changes but not cardiac hypertrophy in aortic-banded rats. Am J Physiol 271:H906-H913;1996.
Ribeiro MO, Antunes E, De Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis: A new model of arterial hypertension. Hypertension 20:298–303;1992.
Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross S, Levi R. NG-methyl-L-arginine, an inhibitor ofL-arginine derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo: A role for nitric oxide in the central regulation of sympathetic tone? Circ Res 70:607–611;1992.
Sasaki R, Watanabe Y, Morishita T. Estimation of the cell number of the heart muscle in normal rats. Tohoku J Exp Med 95:177–84;1968.
Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26:57–60;1970.
Schillaci G, Verdecchia P, Borgroni C, Ciucci A, Zampi I, Battistelli M, Gattobigio R, Sacchi N, Porcellati C. Association between persistent pressure overload and ventricular arrhythmias in essential hypertension. Hypertension 28:284–289;1996.
Slàdek T, Gerová M, Znojil V, Devàt L. Morphometric characteristics of cardiac hypertrophy induced by long-term inhibition of NO synthase. Physiol Res 45:335–338;1996.
Weber KT, Clark WA, Janicki JS, Schroff SG. Physiologic versus pathologic hypertrophy and the pressure overloaded myocardium. J Cardiovasc Pharmacol 10(suppl 6):S37-S49;1987.
Weber KT. Cardiovascular interstitium: Extracelular space of the myocardium. In: Fozzard W, ed. The Heart and Cardiovascular System. New York, Raven Press, 1465–1480;1992.
Weibel ER: Stereological Methods. Practical Methods for Biological Morphometry, vol. 1. London, Academic Press, 1979.
Werchan PM, Summer WR, Gerdes AM, McDonough KH. Right ventricular performance following monocrotaline induced pulmonary hypertension. Am J Physiol 256:H1328-H1336;1989.
Zar JH. Biostatistical Analysis. Upper Saddle River, Prentice-Hall, 1996.
Zierhut W, Zimmer HG, Gerdes AM. Effect of angiotensin converting enzyme inhibition on pressure-induced left ventricular hypertrophy in rats. Circ Res 69:609–617;1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pereira, L.M.M., Mandarim-de-Lacerda, C.A. Quantitative examination of the cardiac myocytes in hypertensive rats under chronic inhibition of nitric oxide synthesis. J Biomed Sci 5, 363–369 (1998). https://doi.org/10.1007/BF02253446
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02253446