Abstract
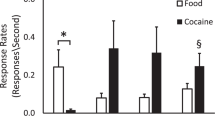
The 2-[14C]deoxyglucose method was used to examine the effects of acute intravenous administration of methamphetamine (0.5–2.5 mg/kg) on rates of local cerebral glucose utilization in freely-moving rats. These effects were correlated with the effects of methamphetamine on locomotor activity assessed simultaneously in the same animals. Methamphetamine administration resulted in widespread dose-dependent increases in glucose utilization within structures of the extrapyramidal motor system. Rates of glucose utilization were positively correlated with locomotor activity in the globus pallidus, substantia nigra reticulata, entopeduncular nucleus, subthalamic nucleus, and the lateral cerebellar cortex. In contrast, within the limbic system alterations in metabolic activity were smaller and more selective. Glucose utilization was increased in the nucleus accumbens at all doses tested, but alterations in glucose utilization in the ventral tegmental area, amygdala, and anterior cingulate were observed only at the highest doses of methamphetamine tested. Significant increases in rates of glucose metabolism were also found in the substantia nigra compacta and in the median and dorsal raphe nuclei. Dopamine and serotonin are depleted in these regions, as well as in the ventral tegmental area where glucose utilization was also increased, following chronic treatment with high doses of methamphetamine. These changes in glucose utilization may be indicative of disturbances in the biochemical processes involved in the neurotoxic effects of methamphetamine.
Similar content being viewed by others
References
Balster RL, Schuster CR (1973) A comparison ofd-amphetamine,l-amphetamine and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav 1:67–71
Chiueh CC, Moore KE (1975a)d-Amphetamine-induced release of brain dopamine. J Pharmacol Exp Ther 192:642–653
Chiueh CC, Moore KE (1975b) Blockade by reserpine of methylphenidate-induced release of brain dopamine. J Pharmacol Exp Ther 193:559–563
Costall B, Marsden CD, Naylor RJ, Pycock CJ (1977) Stereotyped behaviour patterns and hyperactivity induced by amphetamine and apomorphine after discrete 6-hydroxydopamine lesions of the extrapyramidal and mesolimbic nuclei. Brain Res 123:89–111
Crane AM, Porrino LJ (1989) Adaptation of the quantitative 2-[14C]-deoxyglucose method for use in freely-moving rats. Brain Res 499:87–92
Fink JS, GP Smith (1979) Abnormal pattern of amphetamine locomotion after 6-OHDA lesion of anteromedial caudate. Pharmacol Biochem Behav 11:23–30
Heikkila RE, Cabbot FS, Manzino L, Duvoisin RC (1979) Rotational behavior induced by cocaine analogs in rats with unilateral 6-hydroxydopamine lesions of the substantia nigra: dependence upon dopamine uptake inhibition. J Pharmacol Exp Ther 211:189–194
Hubner CB, Koob GF (1990) The ventral pallidum plays a role in mediating cocaine and heroin self-administration. Brain Res 508:20–29
Johanson CE, Balster RL, Bonese K (1976) Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol Biochem Behav 4:45–51
Kadekaro M, Savaki HE, Kutyna FA, Davidsen L, Sokoloff L (1983) Metabolic mapping in the sympathetic ganglia and brain of the spontaneously hypertensive rat. J Cereb Blood Flow Metab 3:460–467
Kameyama T, Nagasaka M (1983) Effects of apomorphine and methamphetamine on a quickly-learned conditioned-suppression response in rats. Neuropharmacology 22:813–817
Kelly PH, Seviour PW, Iversen SD (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res 94:507–522
Kraeuchi K, Rudolph K, Wirz-Justice A, Feer H (1985) Similarities in feeding behavior of chronic methamphetamine treated and withdrawn rats to VMH lesioned rats. Pharmacol Biochem Behav 23:917–920
LeMoal M, Stinus L, Simon H (1979) Increased sensitivity to (+)-amphetamine self-administration by rats following mesocorticolimbic dopamine neurone destruction. Nature 280:156–158
London ED, Wilkerson G, Goldberg SR, Risner ME (1986) Effects ofL-cocaine on local cerebral glucose utilization in the rat. Neurosci Lett 68:73–78
London ED, Wilkerson G, Ori C, Kimes A (1990) Central action of psychostimulants on glucose utilization in extrapyramidal motor areas of the rat brain. Brain Res 512:155–158
Lyness WH, Friedle NM, Moore KE (1979) Destruction of dopamine nerve terminals in nucleus accumbens: effects ond-amphetamine self-administration. Pharmacol Biochem Behav 11:553–556
McCulloch J, Savaki HE, McCulloch MC, Sokoloff L (1980) Retina-dependent activation by apomorphine of metabolic activity in the superficial layer of the superior colliculus. Science 207:313–315
McMillen BA (1983) Two distinct mechanisms for amphetamine-like drugs. TIPS 4:429–432
Missale C, Castelletti L, Govoni S, Spano M, Trabucchi R, Hanbauer I (1985) Dopamine uptake is differentially regulated in rat striatum and nucleus accumbens. J Neurochem 45:51–56
Moore KE, Chiueh CC, Zeldes G (1977) Release of neurotransmitters from the brain in vivo by amphetamine, methylphenidate and cocaine. In: Ellinwood EH, Kilbey MM (eds) Cocaine and other stimulants. Plenum Press, New York, pp 143–160
Nauta WJH, Smith GP, Faull RLM, Domesick VB (1978) Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 3:385–401
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, New York
Pijnenburg AJJ, Honig WMM, Van der Heyden JAM, Van Rossum JM (1976) Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol 35:45–58
Porrino LJ, Lucignani G (1987) Different patterns of energy metabolism associated with high and low doses of methylphenidate. Biol Psychiatry 22:125–138
Porrino LJ, Lucignani G, Dow-Edwards D, Sokoloff L (1984) Dose-dependent effect of acute amphetamine administration on functional brain metabolism in rats. Brain Res 307:311–320
Porrino LJ, Domer FR, Crane AM, Sokoloff L (1988) Selective alterations in cerebral metabolism within the mesocorticolimbic dopaminergic system produced by acute cocaine administration in rats. Neuropsychopharmacology 1:109–118
Raiteri M, Cerrito F, Cervoni AM, Levi G (1979) Dopamine can be released by two mechanism differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther 208:195–202
Ricaurte GA, Schuster CR, Seiden LS (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193:153–163
Rietveld WJ, Korving J, Boom ME, Wirz-Justice A (1987) The circadian control of behavior in the rat affected by the chronic application of methamphetamine. Prog Clin Biol Res 227B:513–517
Roberts DCS, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620
Roberts DCS, Koob GF, Klonoff P, Fibiger HC (1980) Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12:781–787
Ross SF, Renyi AL (1967) Inhibition of the uptake of tritiated catecholamines by antidepressant and related agents. Eur J Pharmacol 2:181–186
Savaki HE, McCulloch J, Kadekaro M, Sokoloff L (1982) Influence of α-receptor blocking agents upon metabolic activity in nuclei involved in central control of blood pressure. Brain Res 233:347–358
Schechter MD, Glennon RA (1985) Cathinone, cocaine and methamphetamine: similarity in behavioral effects. Pharmacol Biochem Behav 22:913–916
Scheel-Krüger J (1971) Comparative studies of various amphetamine analogues demonstrating different interactions with the metabolism of the catecholamines in the brain. Eur J Pharmacol 14:47–59
Schulz EM, Wright JW, Harding JW (1981) Distinction between stereotyped sniffing and licking in rats with methamphetamine and apomorphine. Pharmacol Biochem Behav 15:521–523
Seiden LS, Fischman MW, Schuster CR (1975) Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1:215–219
Seiden LS, Kleven MS (1988) Lack of toxic effect of cocaine on dopamine or serotonin neurons in the rat brain. In: Mechanism of cocaine abuse and toxicity. NIDA Monogr Ser, pp 276–289
Sokoloff L (1986) Cerebral circulation, energy metabolism, and protein synthesis: General characteristics and principles of measurement. In: Phelps ME, Mazziotta J, Schelbert H (eds) Positron emission tomography and autoradiography: principles and applications for the brain and heart. Raven Press, New York, pp 1–71
Sokoloff L, Porrmo EJ (1986) Some fundamental considerations in the application of the deoxyglucose method to pharmacological studies. In: Kriegelstein I (ed) Pharmacology of cerebral ischemia. Amsterdam, Elsevier, pp 65–76
Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Tanabe R, Kaiho M, Yoshii T, Mukaida M, Ishiyama I (1988) Immunohistochemical investigation of topographic localization of phenobarbital and methamphetamine in the rat retina. Exp Eye Res 46:443–449
Wagner GC, Schuster CR, Seiden LS (1981) Neurochemical consequences following administration of CNS stimulants to the neonatal rat. Pharmacol Biochem Behav 14:117–119
Wechsler LR, Savaki H, Sokoloff L (1979) Effects ofd-amphetamine andl-amphetamine on local cerebral glucose utilization in the conscious rat. J Neurochem 32:15–22
Weiner N (1980) Norepinephrine, epinephrine, and the sympathomimetic amines. In: Gilman AG, Goodman LS, Gilman A (eds) The pharmacological basis of therapeutics. Macmillan, New York, pp 138–175
Winn P, Robbins TW (1985) Comparative effects of infusions of 6-hydroxydopamine into nucleus accumbens and anterolateral hypothalamus on the response to dopamine agonists, body weight, locomotor activity and measures of exploration in the rat. Neuropharmacology 24:25–31
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pontieri, F.E., Crane, A.M., Seiden, L.S. et al. Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology 102, 175–182 (1990). https://doi.org/10.1007/BF02245919
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245919