Abstract
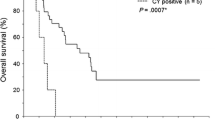
PURPOSE: The aims of this study were to evaluate potential predictors of exfoliated free cancer cells in the peritoneal cavity and to assess intraoperative peritoneal lavage cytology as a prognostic indicator in patients with colorectal cancer. METHODS: From 1985 to 1987, intraoperative peritoneal lavage cytology was performed in 140 patients with colorectal cancer. Among them, 88 patients underwent curative resection and 52 patients had noncurative surgery. Cytology was examined twice,i.e., immediately after opening the peritoneal cavity (precytology) and just before closing the abdomen (postcytology). One hundred milliliters of saline was poured into the peritoneal cavity and it was retrieved by suction after irrigation. Cytologic examination was performed after staining with Papanicolaou, Giemsa, periodic acid-Schiff, and Alcian blue stains. RESULTS: Among the 140 patients examined, the incidence of positive cytology in the prelavage was 15 percent, and that in the postlavage was 9 percent, although it was 16 percent in either lavage. Among patients with curative resection, 10 percent had positive cytology. Seven characteristics were identified as features of tumors which are prone to exfoliate cells into the peritoneal cavity: 1) macroscopic peritoneal dissemination, 2) liver metastasis, 3) more than 20 ml of ascites, 4) ulcerated tumors without definite borders, 5) invasion of the serosal surface or beyond, 6) semiannular or annular shape, and 7) moderate or marked lymphatic invasion. In patients undergoing curative surgery, among these features, circumferential involvement was the only one correlated closely with positive cytology (P<0.02). Positive cytology was associated with a worse outcome. In patients who were resected curatively, the postcytology had a stronger influence on local recurrence than the precytology; the local recurrence rate in patients with positive postcytology was higher than in those with negative postcytology, regardless of the precytology. All patients with cancer cells in the peritoneal cavity at the end of surgery had recurrence. CONCLUSIONS: Seven characteristics were identified as risk factors for exfoliation of cancer cells into the peritoneal cavity in patients with colorectal cancer. These findings may be helpful for the choice of laparoscopic surgery in this era of increasing port-site metastases after laparoscopic procedure. The results of peritoneal lavage cytology at the end of surgery were correlated with the long-term postoperative outcome of colorectal cancer. Thus, meticulous follow-up and possibly adjuvant chemotherapy may be beneficial for patients with free cancer cells in lavage fluid, even after curative surgery.
Similar content being viewed by others
References
Fusco MA, Paluzzi MW. Abdominal wall recurrence after laparoscopic-assisted colectomy for adenocarcinoma of the colon: report of a case. Dis Colon Rectum 1993;36:858–61.
Alexander RJ, Jaques BC, Mitchell KG. Laparoscopically assisted colectomy and wound recurrence [letter]. Lancet 1993;341:249–50.
Lauroy J, Champault G, Risk N, Boutelier P. Metastatic recurrence at the cannula site: should digestive carcinomas still be managed by laparoscopy [abstract]? Br J Surg 1994;81(Suppl):31.
Wexner SD, Cohen SM. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg 1995;82:295–8.
Whelan RL, Sellers GJ, Allendorf JD,et al. Trocar site recurrence is unlikely to result from aerosolization of tumor cells. Dis Colon Rectum 1996;39:S7–13.
Reilly WT, Nelson H, Schroeder G, Wieand HS, Bolton J, O'Connell MJ. Wound recurrence following conventional treatment of colorectal cancer: a rare but perhaps underestimated problem. Dis Colon Rectum 1996;39:200–7.
Vukasin P, Ortega AE, Greene FL,et al. Wound recurrence following laparoscopic colon cancer resection: results of The American Society of Colon and Rectal Surgeons Laparoscopic Registry. Dis Colon Rectum 1996;39:S20–3.
Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res 1994;14:2131–4.
Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol 1978;22:225–9.
Koga S, Kaibara N, Iitsuka Y, Kudo H, Kimura A, Hiraoka H. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol 1984;108:236–8.
Lowe E, McKenna H. Peritoneal washing cytology: a retrospective analysis of 175 gynecological patients. Aust NZ J Obstet Gynaecol 1989;29:55–61.
Ziselman EM, Harkavy SE, Hogan M, West W, Atkinson B. Peritoneal washing cytology: uses and diagnostic criteria in gynecologic neoplasms. Acta Cytol 1984;28:105–10.
Ambrose NS, MacDonald F, Young J, Thompson H, Keighley MR. Monoclonal antibody and cytological detection of free malignant cells in the peritoneal cavity during resection of colorectal cancer—can monoclonal antibodies do better? Eur J Surg Oncol 1989;15:99–102.
Leather AJ, Kocjan G, Savage F,et al. Detection of free malignant cells in the peritoneal cavity before and after resection of colorectal cancer. Dis Colon Rectum 1994;37:814–9.
Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma, 1st ed. Tokyo: Kanehara Shuppan, 1995;11.
Moore GE, Sako K, Kondo T, Badillo J, Burke E. Assessment of the exfoliation of tumor cells into the body cavities. Surg Gynecol Obstet 1961;112:469–74.
Martin JK, Goellner JR. Abdominal fluid cytology in patients with gastrointestinal malignant lesions. Mayo Clin Proc 1986;61:467–71.
Kameyama M, Fukuda I, Imaoka S,et al. Prevention of peritoneal dissemination after colorectal surgery-significance of MMC administration in abdominal cavity. Gan To Kagaku Ryoho 1991;18:1808–11.
Nasu J, Kotake K, Koyama Y, Shimizu H, Hishinuma S, Inada T. Evaluation of peritoneal lavage cytology in patients with advanced colorectal cancer. Jpn J Gastroenterol Surg 1995;28:1991–4.
Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer 1979;44:1476–80.
Yagawa H, Ogawa K, Katsube T, Inaba S. Ogawa T, Ishikawa S. Study of peritoneal lavage cytology during gastric cancer surgery. Nippon Rinsho 1990;51:461–5.
Kunieda K, Saji S, Tsuji K, Kashizuka T, Asano M, Sugiyama Y. Evaluation of intraoperative peritoneal lavage cytology in patients with gastric cancer: with special reference to relationship to area and gross findings of serosal invasion. Nippon Rinsho 1993;54:1167–72.
Imada T. Mechanism by which peritoneal disseminated metastasis develops in gastric cancer. Nippon Geka Gakkai Zasshi 1986;87:593–602.
Skipper D, Cooper AJ, Marston JE, Taylor I. Exfoliated cells andin vitro growth in colorectal cancer. Br J Surg 1987;74:1049–52.
Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg 1984;71:659–63.
Symes MO, Fermor B, Umpleby HC, Tribe CR, Williamson RC. Cells exfoliated from colorectal cancers can proliferate in immune derived mice. Br J Surg 1984;50:423–5.
Ranbarger KR, Johnston WD, Chang JC. Prognostic significance of surgical perforation of the rectum during abdominoperineal resection for rectal carcinoma. Am J Surg 1982;143:186–8.
Slanetz CA Jr. The effect of inadvertent intraoperative perforation on survival and recurrence in colorectal cancer. Dis Colon Rectum 1984;27:792–7.
Zirngibl H, Husemann B, Hermanek P. Intraoperative spillage of tumor cells in surgery for rectal cancer. Dis Colon Rectum 1990;33:610–4.
Author information
Authors and Affiliations
Additional information
Read at the XVth Biennial Congress of The International Society of University Colon and Rectal Surgeons, Singapore, July 2 to 6, 1994.
About this article
Cite this article
Hase, K., Ueno, H., Kuranaga, N. et al. Intraperitoneal exfoliated cancer cells in patients with colorectal cancer. Dis Colon Rectum 41, 1134–1140 (1998). https://doi.org/10.1007/BF02239435
Issue Date:
DOI: https://doi.org/10.1007/BF02239435