Abstract
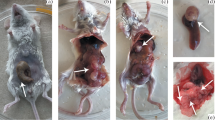
PURPOSE: Local recurrence after colorectal cancer surgery is usually perianastomotic. An experiment was designed to investigate whether free intraluminal cells can penetrate through a colonic anastomosis and thereby cause local recurrence. METHODS: BALB/c and C57/BL mice underwent ascending colotomy followed by watertight anastomosis. Thereafter, CT-26 murine colon carcinoma cells were injected into the cecal lumen 2 cm proximal to the anastomosis of syngeneic BALB/c mice, whereas B-16 murine melanoma cells were injected in the same fashion into C57/BL mice. Control animals without anastomosis received similar injections. Animals were killed 24 hours, 72 hours, and 30 days after surgery and were checked for tumorigenesis. RESULTS: Results of peritoneal fluid cytology were negative after 24 hours, whereas after 72 hours cancer cells were identified in the peritoneal fluid of 80 percent of mice with colotomy and anastomosis compared with 20 percent of control mice. Thirty days after surgery, 11.1 percent of the control BALB/c mice developed pericecal tumor growth, similar to the overall rate of murine melanoma in C57/BL. In mice with anastomoses, perianastomotic tumor growth was observed in 47.5 percent of BALB/c mice (P<0.001) and was correlated with the number of injected cells. Tumor growth reached approximately 75 percent tumor take with high cell densities, whereas in C57/BL mice no difference was found between the experimental and control groups. CONCLUSIONS: The findings suggest that free intraluminal cancer cells of colonic origin may penetrate through watertight anastomoses and implant on the anastomotic or peritoneal surface and initiate tumor growth. This anastomotic penetration is cellmass dependent. The reported experimental model is simple, reproducible, and advantageous for studies of colonic anastomosis.
Similar content being viewed by others
References
Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer 1976;37:2861–5.
McDermott FT, Hughes ES, Pihl E, Johnson WR, Price AB. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg 1985;72:34–7.
Hojo K. Anastomotic recurrence after sphincter-saving resection for rectal cancer: length of distal clearance of the bowel. Dis Colon Rectum 1986;29:11–4.
Polk HC Jr, Spratt JS Jr. Recurrent colorectal carcinoma: detection, treatment, and other considerations. Surgery 1971;69:9–23.
Goligher J, Dukes C, Bussey H. Local recurrence after sphincter saving resections for carcinoma of the rectum and rectosigmoid. Br J Surg 1951;39:199–211.
Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence. Br J Surg 1982;69:613–6.
Rosenberg IL. The aetiology of colonic suture-line recurrence. Ann R Coll Surg Engl 1979;61:251–7.
Gricouroff G. Pathogenesis of recurrences on the suture line following surgical resection for carcinoma of the colon. Cancer 1967;20:673–6.
Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathological correlation and implications for adjuvant therapy. Cancer 1974;34:1278–92.
Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal cancer cells. Br J Surg 1984;71:659–63.
Gordon-Watson C. Origin and spread of cancer of the rectum in relation to surgical treatment. Lancet 1938;1:239–45.
Umpleby HC, Williamson RC. Anastomotic recurrence in large bowel cancer. Br J Surg 1987;74:873–8.
Skipper D, Cooper AJ, Marston JE, Taylor I. Exfoliated cells and in vitro growth in colorectal cancer. Br J Surg 1987;74:1049–52.
Keynes WM. Implantation from the bowel lumen in cancer of the large intestine. Ann Surg 1961;153:357–64.
Yu SK, Cohn I Jr. Tumor implantation on colon mucosa. Arch Surg 1968;96:956–8.
O'Dwyer PJ, Martin EW Jr. Viable intraluminal tumour cells and local/regional tumour growth in experimental colon cancer. Ann R Coll Surg Engl 1989;71:54–6.
Tsujimoto H, Takhashi T, Hagiwara A,et al. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine-labelled cells. Br J Cancer 1995;71:468–72.
Fidler IJ. The evolution of biological heterogeneity in metastatic neoplasms in cancer invasion and metastasis. Biological and therapeutic aspects. New York: Raven Press, 1984.
Netland PA, Zetter BR. Organ-specific adhesion of metastatic tumor cells in vitro. Science 1984;224:1113–5.
Irimura T, Nicolson GL. Carbohydrate chain analysis by lectin binding to electrophoretically separated glycoproteins from murine B16 melanoma sublines of various metastatic properties. Cancer Res 1984;44:791–8.
Nicolson GL, Dulski KM. Organ specificity of metastatic tumor colonization is related to organ-selective growth properties of malignant cells. Int J Cancer 1986;38:289–94.
Author information
Authors and Affiliations
About this article
Cite this article
Kluger, Y., Galili, Y., Yossiphov, J. et al. Model of implantation of tumor cells simulating recurrence in colonic anastomosis in mice. Dis Colon Rectum 41, 1506–1510 (1998). https://doi.org/10.1007/BF02237297
Issue Date:
DOI: https://doi.org/10.1007/BF02237297