Abstract
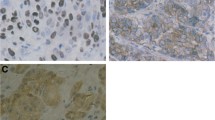
PURPOSE: Because the status of the inherent drug-resistance of colorectal cancers remains obscure, human colorectal cancers with no neoadjuvant therapy were retrospectively investigated regarding the expression of three drug-resistant proteins: metallothionein, glutathione S-transferase-π, and P-glycoprotein. METHODS: Paraffin-embedded tissues of 130 colorectal cancers (Dukes A, 20; B, 49; C, 41; D, 20) obtained by surgical resections from 1982 to 1989 were used. The three proteins were immunostained by the streptavidin-biotin complex method. The immunostaining was judged to be positive if more than 5 percent of cells showed positive staining by use of cell analysis system. The data were compared with clinicopathologic features (Dukes A-D) and patients' prognosis (Dukes A-C). RESULTS: Metallothionein, glutathione S-transferase-π, and P-glycoprotein were positively expressed in 91 (70 percent), 30 (23 percent), and 98 (75 percent), respectively. A total of 120 (86 percent) expressed at least one drug-resistant protein. No intergroup differences were observed between positive and negative expressions of the proteins and their clinicopathologic features except tumor location. Rectal cancers positively expressed P-glycoprotein and three proteins more frequently. Twenty-six (20 percent), 65 (50 percent), and 21 (16 percent) cancers positively expressed one, two, and three proteins, respectively. The disease-free survival rates of patients with Dukes A through C cancer with positive staining for one, two, and three proteins were 100, 94, and 83 percent (at 1 year); 100, 72, and 51 percent (at 3 years); and 94, 66, and 38 percent (at 5 years), respectively (Kaplan-Meier with log-rank test;P = 0.016). In the multivariate Cox analysis, age, Dukes stage, tumor size, and glutathione S-transferase-π were independent prognostic factors. CONCLUSIONS: The patients with concurrent expression of drug-resistant proteins in their cancers had worse prognoses. Examining drug-resistant proteins in colorectal cancers may be useful in selecting adjuvant chemotherapy and in predicting prognosis more accurately.
Similar content being viewed by others
References
Nortenius H. Human Oncology. Vol. 1. Baltimore: Urban & Schwarzenberg, 1988;319–69.
Pinedo HM. Drug resistance, illustration of the complexity of translational research. Steiner award lecture 1995. Int J Cancer 1996;65:561–6.
Basu A, Lazo JS. A hypothesis regarding the protective role of metallothioneins against the toxicity of DNA interactive anticancer drugs. Toxicol Lett 1990;50:123–35.
Kenneth DT. Glutathione-associated enzymes in anticancer resistance. Cancer Res 1994;54:4313–20
Germann UA. P-glycoprotein-A mediator of multidrug resistant in tumor cell. Eur J Cancer 1996;32A:927–44.
Bosch I, Croop J. P-glycoprotein multidrug resistance and cancer. Biochim Biophys Acta 1996;1288:37–54.
Loe DW, Deeley RG, Cole SPC. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer 1996;32A:945–57.
Nitiss JL, Beck WT. Antitopoisomerase drug action and resistance. Eur J Cancer 1996;32A:958–66.
Kille P, Hemmings A, Lunney EA. Memories of metallothionein. Biochim Biophys Acta 1994;1205:151–61.
Aschner M. The functional significance of brain metallothioneins. FASEB J 1996;10:1129–36.
Quaife CJ, Findley SD, Erickson JC,et al. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 1994;33:7250–9.
Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, Lazo JS. Overexpression of metallothionein confers resistance to anticancer drugs. Science 1988;241:1813–5.
Lohrer H, Robson T. Overexpression of metallothionein in CHO cells and its effect on cell killing by ionizing radiation and alkylating agents. Carcinogenesis 1989;10:2279–84.
Waxman DJ. Glutathione S-transferases. Role in alkylating agent resistance and possible target for modulation chemotherapy—A review. Cancer Res 1990;50:6449–54.
Dirven HA, Dictus EL, Broeders NL, Ommen B, Bladeren PJ. The role of human glutathione S-transferase isoenzymes in the formation of glutathione conjugates of the alkylating cytostatic drug thiotepa. Cancer Res 1995;55:1701–6.
Miyazaki M, Kohno K, Saburi Y,et al. Drug resistance to cis-diammine-dichloroplatinum (II) in Chinese hamster ovary cell lines transfected with glutathione S-transferase pi gene. Biochem Biophys Res Commun 1990;166:1358–64.
Okuyama T, Maehara Y, Endo K,et al. Expression of glutathione S-transferase-pi and sensitivity of human gastric cancer cells to cisplatin. Cancer 1994;74:1230–6.
Nishimura T, Newkirk K, Sessions RB,et al. Immunohistochemical staining for glutathione S-transferase predict response to platinum-based chemotherapy in head and neck cancer. Clin Cancer Res 1996;2:1859–1865.
Peters WH, Roelofs HM, Nagengast FM, Tongeren JH. Human intestinal glutathione S-transferase. Biochem J 1989;257:471–6.
Moorghen M, Cairns J, Forrester LM,et al. Enhanced expression of glutathione S-transferases in colorectal carcinoma compared to non-neoplastic mucosa. Carcinogenesis 1991;12:13–7.
Peters WH, Boon CE, Roelofs HM, Webbes T, Nagengast FM, Kremers PG. Expression of drug-metabolizing enzymes and P-170 glycoprotein in colorectal carcinoma and normal mucosa. Gastroenterology 1992;102:448–55.
Satoh K, Kitahara A, Soma Y, Inaba Y, Hatayama I, Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A 1985;82:3964–8.
Sherman M, Campbell AH, Titmuss SA, Kew MC, Kirsch RE. Glutathione S-transferase in human hepatocellular carcinoma. Hepatology 1983;3:170–6.
Kodate C, Fukushi A, Narita T, Kudo H, Soma Y, Sato K. Human placental form of glutathione S-transferase (GST-π) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer 1986;77:226–9.
Tsuchida S, Sekine Y, Shineha R, Nishihira T, Sato K. Elevation of the placental glutathione S-transferase form (GST-π) in tumor tissues and the levels in sera of patients with cancer. Cancer Res 1989;49:5225–9.
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976;455:152–62.
Cordon-Cardo C, O'brien JP, Casals BD, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (p-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 1990;38:1277–87.
Alvarez M, Paull K, Monks A,et al. Generation of a drug resistance profile by quantitation of mdr-1/p-glycoprotein in the cell lines of the national cancer institute anticancer drug screen. J Clin Invest 1995;95:2205–14.
Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A 1987;84:265–9.
Wu L, Smythe AM, Stinson SF,et al. Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res 1992;52:3029–34.
Yang L-Y, Trujillo JM, Siciliano MJ, Kido Y, Siddik ZH, Su Y-Z. Distinct P-glycoprotein expression in two subclones simultaneously selected from a human colon carcinoma cell line by cis-diamminedichloro-platinum (II). Int J Cancer 1993;53:478–85.
Chao CC-K, Huang Y-T, Ma CM, Chou W-Y, Lin-Chao S. Overexpression of glutathione S-transferase and elevation of thiol pools in a multidrug-resistant human colon cancer cell line. Mol Pharmacol 1992;41:69–75.
Satta T, Isobe K, Yamauchi M, Nakasima I, Takagi H. Expression of MDR1 and glutathione S-transferase-π genes and chemosensitivities in human gastrointestinal cancer. Cancer 1992;69:941–6.
Redmond SM, Joncourt F, Buser K,et al. Assessment of p-glycoprotein, glutathione-based detoxifying enzymes and O6-alkylguanine-DNA alkyltransferase as potential indicators of constructive drug resistance in human colorectal tumors. Cancer Res 1991;51:2092–7.
Dukes CE. The classification of cancer of the rectum. J Pathol 1932;35:323–32.
Hsu SM, Raine L, Fanger H. Use of avidin-biotinperoxidase technique: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–81.
Sincrope FA, Hart J, Brasitus TA, Michelassi F, Lee J, Safa AR. Relationship of p-glycoprotein and carcinoembryonic antigen expression in human colon carcinoma to local invasion, DNA ploidy, and disease relapse. Cancer 1994;74:2908–17.
Hishikawa Y, Abe S, Kinugasa S,et al. Overexpression of metallothionein correlates with chemoresistance to cisplatin and prognosis in esophageal cancer. Oncology 1997;54:342–7.
Monden N, Abe S, Sutoh I, Hishikawa Y, Kinugasa S, Nagasue N. Prognostic significance of the expressions of metallothionein, glutathione S-transferase-π, and P-glycoprotein in curatively resected gastric cancer. Oncology 1997;54:391–9.
Dolfini E, Dasdia T, Arancia G,et al. Characterization of clonal colon adenocarcinoma line intrinsically resistant to doxorubicin. Br J Cancer 1997;76:67–76.
Dolfini E, Dasdia T, Perletti G,et al. Analysis of calcium-dependent protein kinase-C isoenzymes in intrinsically resistant cloned lines of LoVo cells. Reversal of resistance by kinase inhibitor 1-(5-isoquinolinylsulfonyl)2-methylpiperazine. Anticancer Res 1993;13:1123–8.
Slovak ML, Coccia M, Meltzer PS, Trent JM. Molecular analysis of two human doxorubicin-resistant cell lines: evidence for differing multidrug resistance mechanisms. Anticancer Res 1991;11:423–8.
Dong Z, Ward NE, Fan D, Gupta KP, O'brien CA. In vitro model for intrinsic drug resistance: effects of protein kinase C activators on the chemosensitivity of cultured human colon cancer cells. Mol Pharmacol 1991;39:563–9.
Friedman E, Isaksson P, Rafter J, Marian B, Winawer S, Newmark H. Fecal diglycerides as selective endogenous mitogen for premalignant and malignant human colonic epithelial cells. Cancer Res 1989;49:544–8.
Bakka A, Endersen L, Johnsen AB, Edminson PD, Rugstad HE. Resistance against cis-dichlorodiamineplatinum in cultured cells with a high content of metallothionein. Toxicol Appl Pharmacol 1981;61:215–26.
Tohyama C, Suzuki JS, Hemelraad J, Nishimura N, Nishimura H. Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology 1993;18:1193–201.
Nagel WW, Vallee BL. Cell cycle regulation of metallothionein in human colonic cancer cells. Proc Natl Acad Sci U S A 1995;92:579–83.
Woo ES, Lazo JS. Nucleocytoplasmic functionality of metallothionein. Cancer Res 1997;57:4236–41.
Öfner D, Maier H, Riedmann B,et al. Immunohistochemical metallothionein expression in colorectal adenocarcinoma: correlation with tumor stage and patient survival. Virchows Arch 1994;425:491–7.
Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci 1992;17:433–8.
Ban N, Takahashi Y, Takayama T,et al. Transfection of glutathione S-transferase (GST)- π antisense complementary DNA increase the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res 1996;56:3577–82.
Kantor RR, Giardina SL, Bartolazzi A,et al. Monoclonal antibodies to glutathione S-transferase π-immunohistochemical analysis of human tissues and cancers. Int J Cancer 1991;47:193–201.
Ranganathan S, Tew KD. Immunohistochemical localization of glutathione S-transferases-α, μ, and π in normal tissue and carcinomas from human colon. Carcinogenesis 1991;12:2383–7.
McKay JA, Murray GI, Weaver RJ, Ewen SW, Melvin WT, Burke MD. Xenobiotic metabolising enzyme expression in colonic neoplasia. Gut 1993;34:1234–9.
Mulder TP, Verspaget HW, Sier CF,et al. Glutathione S-transferase-π in colorectal tumors is predictive for overall survival. Cancer Res 1995;55:2696–702.
Goldstein LJ, Galski H, Fojo A,et al. Expression of multidrug resistance gene in human cancers. J Natl Cancer Inst 1989;81:116–24.
Weinstein RS, Jakate SM, Dominguez JM,et al. Relationship of the expression of the multidrug resistance gene product (P-Glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res 1991;51:2720–6.
Pirker R, Wallner J, Gsur A,et al. MDR1 gene expression in primary colorectal carcinomas. Br J Cancer 1993;68:691–4.
Mikami K, Naito M, Tomida A, Yamada M, Sirakusa T, Tsuruo T. DT-diaphorase as a critical determinant of sensitivity to mitomycin C in human colon and gastric carcinoma cell line. Cancer Res 1996;56:2823–6.
Kinsella AR, Smith D, Pickard M. Resistance to chemotherapeutic antimetabolites: a functional of salvage pathway involvement and cellular response to DNA damage. Br J Cancer 1997;75:935–45.
Benhattar J, Cerottini JP, Saraga E, Metthez G, Givel J-C. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer 1996;69:190–2.
Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res 1996;56:2422–7.
Alas MM, Aebi S, Fink D, Howell SB, Los G. Loss of DNA mismatch repair: effect on the rate of mutation to drug resistance. J Natl Cancer Inst 1997;89:1537–41.
Satoh M, Cherian G, Imura N, Shimizu H. Modulation of resistance to anticancer drugs by inhibition of metallothionein synthesis. Cancer Res 1994;54:5255–7.
Hall A, Robson CN, Hickson ID, Harris AL, Proctor SJ, Cattan AR. Possible role of inhibition of glutathione S-transferase in the partial reversal of chlorambucil resistance by indomethacin in a Chinese hamster ovary cell line. Cancer Res 1989;54:6265–8.
Stein U, Walther W, Shoemaker RH. Reversal of multidrug resistance by transduction of cytokine genes into human colon carcinoma cells. J Natl Cancer Inst 1996;88:1383–92.
Author information
Authors and Affiliations
About this article
Cite this article
Sutoh, I., Kohno, H., Nakashima, Y. et al. Concurrent expressions of metallothionein, glutathione S-transferase-π, and P-glycoprotein in colorectal cancers. Dis Colon Rectum 43, 221–232 (2000). https://doi.org/10.1007/BF02236987
Issue Date:
DOI: https://doi.org/10.1007/BF02236987