Abstract
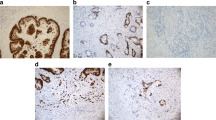
PURPOSE: The aim of this study was to determine the value of DCC (deleted in colorectal cancer) protein for predicting metachronous distant metastases after curative surgery for rectal cancer. The DCC protein—for which a gene has been located on chromosome 18q—has recently been reported to have a prognostic value in colorectal cancer. This finding might have implications for treatment of International Union Against Cancer Stage II rectal carcinoma, in which distant metastases will develop in 14 percent of patients despite optimal surgery. METHODS: Paraffin-embedded tissues from 85 patients who developed distant metastases, but no local recurrence, after curative surgery for rectal cancer were matched with 85 samples from patients who remained disease-free. Matching criteria were tumor stage, age, gender, and date of surgery. Expression of DCC protein was assessed using immunohistochemistry. End points of follow-up were recurrence of disease and death. Mean follow-up was 9.6 years. No patient received either local or systemic adjuvant therapy. RESULTS: The DCC protein was found to be expressed in 64.9 percent of tumor samples. Nonexpression of DCC protein had an negative influence on survival (P=0.03). For all tumor stages together, sensitivity of the test for subsequent occurrence of distant metastases was 42 percent and specificity was 71 percent. In Stage II cancers, the positive predictive value was 19 percent, and the negative predictive value was 88 percent. CONCLUSIONS: Our results confirm that DCC protein is a useful prognostic marker in patients with rectal carcinomas, but the positive predictive value of DCC protein for occurrence of metachronous metastases does not appear to be sufficient to justify adjuvant therapeutic measures in Stage II rectal cancer.
Similar content being viewed by others
References
Hedrick L, Cho KR, Fearon ER, Wu TC, Kinzler KW, Vogelstein B. The DCC gene product in cellular differentiation, colorectal tumorigenesis. Genes Dev 1994;8:1174–83.
Peinado MA, Malkhosyan S, Velasquez A, Perucho M. Isolation and characterization of allelic losses and amplifications in colorectal tumors by arbitrarily primed polymerase chain reaction. Proc Natl Acad Sci U S A 1996;89:10065–9.
Tannapfel A, Köckerling F, Katalinic A, Wittekind C. Expression of nm23-H1 predicts lymph node involvement in colorectal carcinoma [published erratum appears in Dis Colon Rectum 1995;38:802. Dis Colon Rectum 1995;38:651–4.
Cho KR, Vogelstein B. Suppressor gene alterations in the colorectal adenoma-carcinoma sequence. J Cell Biochem Suppl 1992;16G:137–41.
Jiang WG, Puntis MC, Hallett MB. Molecular and cellular basis of cancer invasion and metastatic implications for treatment. Br J Surg 1994;81:1576–90.
Fearon ER, Cho KR, Nigro JM. Identification of a chromose 18q that is altered in colorectal cancer. Science 1990;247:49–56.
Klingelhutz AJ, Hedrick L, Cho KR, McDougall JK. The DCC gene suppresses the malignant phenotype of transformed human epithelial cells. Oncogene 1995;10:1581–6.
Jen J, Kim H, Piantadosi S. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 1994;331:213–21.
Shibata D, Reale MA, Lavin P,et al. The DCC protein and prognosis in colorectal cancer. N Engl J Med 1996;335:1727–32.
Köckerling F, Reymond MA, Altendorf-Hofmann A, Hohenberger W. The influence of surgery on metachronous distant metastases and survival in rectal cancer. J Clin Oncol 1998;16:324–9.
Sobin LH, Wittekind C (UICC), eds. TNM classification of malignant tumors. 5th ed. Baltimore: Wiley-Liss, 1997.
Umpleby HC, Bristol JB, Rainey JB, Williamson RC. Survival of 727 patients with single carcinomas of the large bowel. Dis Colon Rectum 1984;27:803–10.
Olson RM, Perencevich NP, Malcolm AW, Chaffey JT, Wilson RE. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer 1980;45:2969–74.
Gall FP, Hermanek P. Wandel und derzeitiger Stand der chirurgischen Behandlung des colorectalen Carcinoms. Chirurg 1992;63:227–34.
Pilipshen SJ, Heilweil M, Quan HQ, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984;53:1354–62.
Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg 1979;190:350–60.
Carlsson U, Lasson A, Ekelund G. Recurrence rates after curative surgery for rectal carcinoma, with special reference to their accuracy. Dis Colon Rectum 1987;30:431–4.
McDermott FT, Hughes ES, Pihl E, Johnson WR, Price AB. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg 1985;72:34–7.
Philips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following “curative” surgery for large bowel cancer. I. The overall picture. Br J Surg 1984;761:12–6.
Hermanek P, Wiebelt H, Staimmer D, Riedel St, for The German Study Group Colo-Rectal Carcinoma (SGCRC). Prognostic factors of rectum carcinoma: experience of the German Multicenter Study SGCRC. Tumori 1995;81(Suppl 1):60–4.
Hohenberger W. The effect of specialization or organization of rectal cancer surgery. In: Søreide O, ed. Rectal cancer surgery: optimization—standardisation—documentation. Heidelberg: Springer-Verlag, 1996.
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1477–82.
Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg 1982;82:613–6.
NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444–50.
Author information
Authors and Affiliations
Additional information
Supported by Life Sciences International Inc., Frankfurt, Germany. Read at the meeting of the Swiss Surgical Society, Davos, Switzerland, June 12 to 14, 1997.
About this article
Cite this article
Reymond, M.A., Dworak, O., Remke, S. et al. DCC protein as a predictor of distant metastases after curative surgery for rectal cancer. Dis Colon Rectum 41, 755–760 (1998). https://doi.org/10.1007/BF02236264
Issue Date:
DOI: https://doi.org/10.1007/BF02236264