Abstract
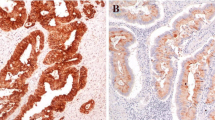
PURPOSE: We tried to elucidate the effects of sulindac on human colorectal carcinoma. METHODS: Sulindac (300 mg/day) was administered for two weeks before operation to 33 patients with sporadic colorectal carcinoma (Sulindac Group). Resected specimens were used to detect apoptosis by terminal dUTP nick end labeling and transforming growth factor (TGF)-β1 expression by immunohistochemistry. The results were compared with those from the historical Control Group. Twenty-nine available preoperative biopsies taken from carcinomas before sulindac prescription and 22 concurrent colorectal adenomas (9 and 13 in Sulindac and Control Groups, respectively) in the resected specimen were also examined regarding TGF-β1 expression. RESULTS: In the resected carcinomas and adenomas, more frequent apoptosis and higher TGF-β1 scores were observed in the Sulindac Group than in the Control Group. Overexpression of TGF-β1 and apoptosis occurred in the same region in adenomas but not in carcinomas. A positive correlation between TGF-β1 scores and apoptotic frequency was found in adenomas (P=0.01,ρ=0.91) but not in carcinomas (P=0.89,ρ=0.03). CONCLUSION: We conclude that sulindac induces apoptosis in human colorectal carcinomas as well as in adenomas. Also, one of the antineoplastic effects of sulindac might be mediated by upregulating TGF-β1 expression, particularly in colorectal adenomas.
Similar content being viewed by others
References
Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991;325:1593–96.
Shiff SJ, Qiao L, Tsai L-L, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest 1995;96:491–503.
Goldberg Y, Nassif II, Pittas A, Tsai LL. The antiproliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppresser and cell cycle-regulatory proteins. Oncogene 1996;12:893–901.
Qiao L, Shiff SJ, Rigas B. Sulindac sulfide inhibits the proliferation of colon cancer cells: diminished expression of the proliferation markers PCNA and Ki-67. Cancer Lett 1997;115:229–34.
Rao CV, Rivenson A, Simi B. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal antiinflammatory agent. Cancer Res 1995;55:1464–72.
Piazza GA. Alberts DS, Hixson LJ. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res 1997;57:2909–15.
Boolbol SK, Dannenberg AJ, Chadburn A. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 1996;56:2556–60.
Waddell WR, Ganser GF, Cerise EJ, Loughry RW. Sulindac for polyposis of the colon. Am J Surg 1989;157:175–9.
Giardiello FM, Hamilton SR, Krush AJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993;328:1313–6.
Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut 1997;40:344–9.
Kelloff GJ, Crowell JA, Boone CW. Clinical development plan: sulindac. J Cell Biochem 1994;(Suppl)20:S240–51.
Eberhart CE, DuBois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology 1995;109:285–301.
Eberhart C-E, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1944;107:1183–8.
Sheng H, Shao J, O'Mahony CA. Transformation of intestinal epithelial cells by chronic TGF-β1 treatment results in downregulation of type II TGF-β receptor and induction of cyclooxugenase-2. Oncogene 1999;18:855–67.
Shao J, Sheng H, Armandla R. Coordinate regulation of cyclooxygenase-2 and TGF-β1 in replication error-positive colon cancer and azoxymethane-induced rat colonic tumors. Carcinogenesis 1999;20:185–91.
Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β). Growth Factors 1993;8:1–9.
Sinicrope FA, Pazdur R, Levin B. Phase I trial of sulindac plus 5-fluorouracil and levamisole: potential adjuvant therapy for colon carcinoma. Clin Cancer Res 1996;2:37–41.
Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res 1997;57:1301–5.
Verheul HM, Panigrahy D, Yuan J, D'Amato RJ. Combination oral antiangiogenic therapy with thalidomide and sulindac inhibits tumor growth in rabbits. Br J Cancer 1999;79:114–8.
Piazza GA. Rahm AK, Finn TS. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res 1997;57:2452–9.
Piazza G, Rahm A, Pamukcu R, Ahnen D. Induction of apoptosis by sulindac metabolites involves a p53 and bcl-2 independent mechanism and dose not require cell cycle arrest [abstract]. Gastroenterology 1996;110:577.
Arber N, Han EK-H, Sgambato A. A K-ras oncogene increases resistance to sulindac-induced apoptosis in rat enterocytes. Gastroenterology 1997;113:1892–1900.
Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA 1998;95:681–6.
McEntee MF, Chiu C-H, Whelan J. Relationship ofβ-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis 1999;20:635–40.
Waddell WR. Stimulation of apoptosis by sulindac and piroxicam. Clin Sci 1998;95:385–8.
Robson H, Anderson E, James RD, Schofield PF. Transforming growth factorβ1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer 1996;74:753–8.
Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-β1 in patients with colorectal carcinoma. Its association with tumor progression and its significant decrease after curative surgical resection. Cancer 1999;85:554–61.
Brattain MG, Markowitz SD, Willson JK. The type II transforming growth factor-β receptor as a tumor-suppresser gene. Curr Opin Oncol 1996;8:49–53.
Markowitz S, Wang J, Myeroff L. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995;268:1336–8.
Akiyama Y, Iwanaga R, Saitoh K. Transforming growth factorβ type II receptor gene mutations in adenomas from hereditary nonpolyposis colorectal cancer. Gastroenterology 1997;112:33–9.
Samowitz WS, Slattery ML. Transforming growth factor-β receptor type 2 mutations and microsatellite instability in sporadic colorectal adenomas and carcinomas. Am J Pathol 1997;151:33–5.
Akiyama Y, Iwanaga R, Ishikawa T. Mutations of the transforming growth factor-β type II receptor gene are strongly related to sporadic proximal colon carcinomas with microsatellite instability. Cancer 1996;78:2478–84.
Imai Y, Tsurutani N, Oda H, Inoue T, Ishikawa T. Genetic instability and mutation of the TGF-β-receptor-II gene in ampullary carcinomas. Int J Cancer 1998;76:407–11.
Hague A, Bracey TS, Hicks DJ, Reed JC, Paraskeva C. Decreased levels of p26-Bcl-2, but not p30 phosphorylated Bcl-2, precede TGFβ1-induced apoptosis in colorectal adenoma cells. Carcinogenesis 1998;19:1691–5.
Miyaki M, Iijima T, Konishi M. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 1999;18:3098–103.
Author information
Authors and Affiliations
About this article
Cite this article
Masunaga, R., Kohno, H., Dhar, D.K. et al. Enhanced apoptosis and transforming growth factor-β1 expression in colorectal adenomas and carcinomas after sulindac therapy. Dis Colon Rectum 44, 1008–1015 (2001). https://doi.org/10.1007/BF02235490
Issue Date:
DOI: https://doi.org/10.1007/BF02235490