Abstract
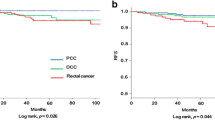
PURPOSE: The aim of this study was to determine whether tumor location proximal or distal to the splenic flexure is associated with distinct molecular patterns and can predict clinical outcome in a homogeneous group of patients with Dukes B (T3–T4, N0, M0) colorectal cancer. It has been hypothesized that proximal and distal colorectal cancer may arise through different pathogenetic mechanisms. Althoughp53 and Ki-ras gene mutations occur frequently in distal tumors, another form of genomic instability associated with defective DNA mismatch repair has been predominantly identified in the proximal colon. To date, however, the clinical usefulness of these molecular characteristics remains unproven. METHODS: A total of 126 patients with a lymph node-negative sporadic colon or rectum adenocarcinoma were prospectively assessed with the endpoint of death by cancer. No patient received either radiotherapy or chemotherapy. p53 protein was studied by immunohistochemistry using DO-7 monoclonal antibody, andp53 and Ki-ras gene mutations were detected by single strand conformation polymorphism assay. RESULTS: During a mean follow-up of 67 months, the overall five-year survival was 70 percent. Nuclearp53 staining was found in 57 tumors (47 percent), and was more frequent in distal than in proximal tumors (55vs. 21 percent; chi-squared test,P<0.001). For the whole group, p53 protein expression correlated with poor survival in univariate and multivariate analysis (log-rank test,P=0.01; hazard ratio=2.16; 95 percent confidence interval=1.12−4.11,P=0.02). Distal colon tumors and rectal tumors exhibited similar molecular patterns and showed no difference in clinical outcome. In comparison with distal colorectal cancer, proximal tumors were found to be statistically significantly different on the following factors: mucinous content (P=0.008), degree of histologic differentiation (P=0.012), p53 protein expression, and gene mutation (P=0.001 and 0.01 respectively). Finally, patients with proximal tumors had a marginally better survival than those with distal colon or rectal cancers (log-rank test,P=0.045). CONCLUSION: In this series of Dukes B colorectal cancers, p53 protein expression was an independent factor for survival, which also correlated with tumor location. Eighty-six percent ofp53-positive tumors were located in the distal colon and rectum. Distal colon and rectum tumors had similar molecular and clinical characteristics. In contrast, proximal neoplasms seem to represent a distinct entity, with specific histopathologic characteristics, molecular patterns, and clinical outcome. Location of the neoplasm in reference to the splenic flexure should be considered before group stratification in future trials of adjuvant chemotherapy in patients with Dukes B tumors.
Similar content being viewed by others
References
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics 1996. CA Cancer J Clin 1996;46:5–27.
Obrand DI, Gordon PH. Continued change in the distribution of colorectal carcinoma. Br J Surg 1998;85:246–8.
Levi F, Randinbison L, La Vecchia C. Trends in subsite distribution of colorectal cancers and polyps from the Vaud Cancer Registry. Cancer 1993;72:46–50.
Jass JR. Subsite distribution and incidence of colorectal cancer in New Zealand 1974–1983. Dis Colon Rectum 1991;34:56–9.
Distler P, Holt PR. Are right- and left-sided neoplasms distinct tumors? Dig Dis 1997;15:302–11.
Pocard M, Salmon RJ, Muleris M,et al. Two colons-two cancers? Proximal or distal adenocarcinomas: arguments for two types of carcinogenesis [in French]. Bull Cancer 1995;82:10–21.
Vogelstein B, Fearon ER, Hamilton SR,et al. Genetic alterations during colorectal tumor development. N Engl J Med 1988;319:525–32.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67.
Levine AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature 1991;351:453–6.
Bedenne L, Faivre J, Boutron MC,et al. Adenocarcinoma sequence or de novo carcinogenesis? A study of adenomatous remnants in a population-based series of large bowel cancers. Cancer 1992;69:883–8.
Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–9.
Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994;145:148–56.
Corman ML. Colon and rectal surgery, 4th ed. Philadelphia: Lippincott-Raven, 1998:733–862.
Chung DC. Molecular prognostic markers and colorectal cancer: the search goes on. Gastroenterology 1998;114:1330–8.
Chaubert P, Bautista D, Benhattar J. An improved method for rapid screening of DNA mutations by non-radioactive single-strand conformation polymorphism procedure. Biotechniques 1993;15:586.
Sugarbaker JP, Gunderson LL, Wites RE. Colorectal cancer. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: JB Lippincott, 1985:800–3.
Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990;113:779–88.
Delattre O, Olschwang S, Law DJ,et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet 1989;8659:353–6.
Jernvall P, Makinen M, Karttunen T, Makela J, Vihko P. Conserved region mutations of the p53 gene are concentrated in distal colorectal cancers. Int J Cancer 1997;74:97–101.
Manne U, Weiss HL, Myers RB,et al. Nuclear accumulation of p53 in colorectal adenocarcinoma: prognostic importance differs with race and location of the tumor. Cancer 1998;83:2456–67.
Bosari S, Viale G. The clinical significance of p53 aberrations in human tumors. Virchows Arch 1995;427:229–41.
Leahy DT, Salman R, Mulcahy H, Sheahan K, O'Donoghue DP, Parfray NA. Prognostic significance of p53 abnormalities in colorectal carcinoma detected by PCR-SSCP and immunohistochemical analysis. J Pathol 1996;180:364–70.
Remvikos Y, Tominaga O, Hammel P,et al. Increased p53 protein content of colorectal tumors correlates with poor survival. Br J Cancer 1992;66:758–64.
Ahnen DJ, Feigl P, Quan G,et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 1998;58:1149–58.
Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Swedish Rectal Cancer Trial. Eur J Surg 1996;162:397–402.
Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980–7.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993;74:957–67.
Gryfe R, Kim H, Hsieh ET,et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
Goichi T, Makoto O, Masayuku M,et al. The mutual exclusiveness of presence of the microsatellite instability and alteration of p53 in sporadic colorectal cancer [abstract]. Gastroenterology 2000;118(Suppl 2):A59.
Author information
Authors and Affiliations
Additional information
Supported by a grant from the SICPA foundation and by the Swiss National Science Foundation
Read at the meeting of The American Society of Colon and Rectal Surgeons, Boston, Massachusetts, June 24 to 29, 2000.
About this article
Cite this article
Gervaz, P., Bouzourene, H., Cerottini, JP. et al. Dukes B colorectal cancer. Dis Colon Rectum 44, 364–372 (2001). https://doi.org/10.1007/BF02234734
Issue Date:
DOI: https://doi.org/10.1007/BF02234734