Abstract
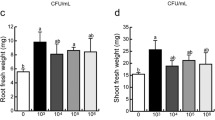
The possible involvement of IAA in the effect thatAzospirillum brasilense has on the elongation and morphology ofPanicum miliaceum roots was examined by comparing in a Petri dish system the effects of inoculation with a wild strain (Cd) with those of an IAA-overproducing mutant (FT-326). Both bacterial strains produced IAA in culture in the absence of tryptophan. At the stationary growth phase, production of IAA by FT-326 wasca. 12 times greater than that of Cd. When inoculation was made with bacterial concentrations higher than, 106 colony forming units ml−1 (CFU ml−1), both strains inhibited root elongation to the same extent. At lower concentrations Cd enhanced elongation, by 15–20%, while FT-326 was ineffective. Both strains promoted root-hair development, and root-hairs were produced nearer the root tip the higher the bacterial concentration (e. g. root elongation region was reduced). Effects of FT-326 on root-hair development were greater than those of Cd. Acidified ether extracts of Cd and FT-326 cultures had inhibitory or promoting effects on root elongation depending on the dilution applied. At low dilutions, extracts from FT-326 were more inhibitory for elongation than those from Cd. At higher dilutions root elongation was promoted, but FT-326 extracts had to be more diluted than those from Cd. Dilutions that promoted root elongation contained supra-optimal concentrations of IAA, 1–3 orders of magnitude higher than those required for optimal enhancement by synthetic IAA. It is suggested that the bacteria produce in culture an IAA-antagonist or growth inhibitor that decreases the effectiveness of IAA action. The large variability reported for the effects ofAzospirillum on root elongation could be the result of the opposite effects on root elongation of IAA and other compounds, produced by the bacteria.
Similar content being viewed by others
References
Barton L L, Johnson G V and Orbok Miller S 1986 The effect ofAzospirillum brasilense on iron absorption and translocation by sorghum. J. Plant Nutr. 9, 557–565.
Boddey R M and Döbereiner J 1982 Association ofAzospirillum and other diazotrophs with tropical graminae.In 12th, International Congress of Soil Science, New Delhi, International Society of Soil Science, FAO, Rome. Vol. 1, 28–47.
Cohen E, Okon Y, Kigel J, Nur I and Henis Y 1980 Increase in dry weight and total nitrogen content inZea mays andSetaria italica associated with nitrogen-fixingAzospirillum spp Plant Physiol, 66, 746–749.
Echline P 1971 Preparation of labile biological material for examination in the scanning electron microscope.In Scanning Electron Microscopy. Ed. V H Haywood. pp 307–315. Academic Press, NY.
Giller K E and Day J M 1985 Nitrogen fixation in the rhizosphere: Significance in natural and agricultural systems.In Biological Interaction in Soil. Ed. A H Fitter. pp 137–147. Blackwell Scientific Publications, Oxford.
Hartmann A, Fuseder A and Klingmüller W, 1983a Mutants ofAzospirillum affected in nitrogen fixation and auxin production.In Azospirillum II. Genetics, Physiology, Ecology. Ed. W Klingmüller. pp 78–87. Birkhauser Verlag, Basel.
Hartmann A, Singh M and Klingmüller W 1983b Isolation and characterization ofAzospirillum mutants excreting high amounts of indoleacetic acid. Can. J. Microbiol. 29, 916–923.
Iino M, Yu R S T and Carr D J 1980 Improved procedure for the estimation of nanogram quantities of indole-3-acetic acid in plant extracts using the indolo-pyrone fluorescence method. Plant Physiol. 66, 1099–1105.
Inbal E and Feldman M 1982 The response of a hormonal mutant of common wheat to bacteria of the genusAzospirillum. Isr. J. Bot. 31, 257–263.
Kapulnik Y, Okon Y, Kigel J, Nur I and Henis Y 1981 Effects of temperature, nitrogen fertilization and plant age on nitrogen fixation bySetaria italica inoculated withAzospirillum brasilense (strain Cd) Plant Physiol. 68, 340–343.
Kapulnik Y, Gafny R and Okon Y 1984 Effect ofAzospirillum spp. inoculation on root development and NO3 uptake in wheat (Triticum aestivum cv. Miriam) in hydroponic systems. Can. J. Bot. 63, 627–631.
Kapulnik Y, Okon Y and Henis Y 1985 Changes in root morphology of wheat caused byAzospirillum inoculation. Can. J. Microbiol. 31, 881–887.
Lin W, Okon Y and Hardy R W F 1983 Enhanced mineral uptake byZea mays andSorghum bicolor roots inoculated withAzospirillum brasilense. Appl. and Environ. Microbiol. 45, 1775–1779.
Morgenstern E and Okon Y 1987 The effect ofAzospirillum brasilense and auxin on root morphology in seedlings ofSorghum bicolor x Sorghum sudanense. Arid Soil Res. Rehabil. 1, 115–127.
Okon Y 1985Azospirillum as a potential inoculant for agriculture. Trends in Biotechnology 3, 223–228.
Okon Y and Kapulnik Y 1986 Development and function ofAzospirillum-inoculated roots. Plant and Soil 90, 3–16.
Patriquin D G, Döbereiner J and Jain D K 1983 Sites and processes of association between diazotrophs and grasses. Can. J. Microbiol. 29, 900–915.
Reynders L and Vlassak M 1979 Conversion of tryptophan to indoleacetic acid byAzospirillum brasilense. Soil Biol. Biochem. 11, 547–548.
Sarig S, Okon Y and Blum A 1985 Improvement of growth and yield of non-irrigatedSorghum bicolor byAzospirillum inoculation.In Nitrogen Fixation Research Progress. Eds. H J Evans, P J Bottomley and W E Newton. pp 707. Martinus Nijhoff, Dordrecht.
Scott T K 1972 Auxin and roots. Annu. Rev. Plant Physiol. 23, 235–258.
Stoessl A and Venis M A 1970 Determination of submicrogram levels of indole-3-acetic acid: a new, highly specific method. Anal. Biochem. 34, 344–351.
Tien T M, Gaskins M H and Hubbell D H 1979 Plant growth substances produced byAzospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.) Appl. Environ. Microbiol. 37, 1016–1024.
Torrey J G 1976 Root hormones and plant growth. Annu. Rev. Plant Physiol. 27, 435–459.
Umali-Garcia M, Hubbell D H, Gaskins M H and Dazzo F B 1980 Association ofAzospirillum with grass roots. Appl. Environ. Microbiol. 39, 219–226.
Wightman F, Schneider E A and Thimann K V 1980 Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea, roots. Physiol. Plant. 49, 304–314.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harari, A., Kigel, J. & Okon, Y. Involvement of IAA in the interaction betweenAzospirillum brasilense andPanicum miliaceum roots. Plant Soil 110, 275–282 (1988). https://doi.org/10.1007/BF02226808
Issue Date:
DOI: https://doi.org/10.1007/BF02226808