Abstract
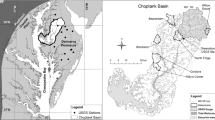
Relationships between surface-water discharge, water chemistry, and watershed geology were investigated to evaluate factors affecting the sensitivity of drainage waters in the Adirondack region of New York to acidification by atmospheric deposition. Instantaneous discharge per unit area was derived from relationships between flow and staff-gage readings at 10 drainage basins throughout the region. The average chemical composition of the waters was assessed from monthly samples collected from July 1982 through July 1984. The ratio of flow at the 50-percent exceedence level to the flow at the 95-percent exceedence level of flow duration was negatively correlated with mean values of alkalinity or acid-neutralizing capacity (ANC), sum of basic cations (SBC), and dissolved silica, for basins containing predominantly aluminosilicate minerals and little or no carbonate-bearing minerals. Low ratios are indicative of systems in which flow is predominately derived from surface- and ground-water storage, whereas high ratios are characteristic of watersheds with variable flow that is largely derived from surface runoff.
In an evaluation of two representative surface-water sites, concentrations of ANC, SBC, and dissolved silica, derived primarily from soil mineral weathering reactions. decreased with increasing flow. Furthermore, the ANC was highest at low flow when the percentage of streamflow derived from ground water was maximum. As flow increased, the ANC decreased because the contribution of dilute surface runoff and lateral flow through the shallow acidic soil horizons to total flow increased. Basins having relatively high ground-water contributions to total flow, in general, have large deposits of thick till or stratified drift. A major factor controlling the sensitivity of these streams and lakes to acidification is the relative contribution of ground water to total discharge.
Similar content being viewed by others
References
American Public Health Association. 1975. Standard methods for the analysis of water and wastewater [14th ed.]. Washington, D.C., American Public Health Association.
Chen, C. W., S. A. Gherini, N. E. Peters, P. S. Murdoch, R. M. Newton, and R. A. Goldstein. 1984. Hydrologic analysis of acidic and alkaline lakes. Water Resources Research 20: 1875–1882.
Cogbill, C. V., and G. E. Likens. 1974. Acid precipitation in the northeastern United States. Water Resources Research 10: 1133–1137.
Colquhoun, J. R., J. Symula, M. H. Pfeiffer, and J. Feurer. 1981. Preliminary report of stream sampling for acidification studies, 1980. New York State Department of Envirionmental Conservation technical report 81-2.
Colquhoun, J. R., J. Symula, and R. W. Karcher, Jr. 1982. Report of Adirondack sampling for stream acidification studies, 1981 supplement. New York State Department of Environmental Conservation technical report 82-3.
Colquhoun, J. R., W. A. Kretser, and M. H. Pfeiffer. 1984. Acidity Status update of lakes and streams in New York State. New York State Department of Environmental Conservation.
Cronan, C. S., J. C. Conlan, and S. Skibinski. 1987. Forest vegetation in relation to surface water chemistry in the North Branch of the Moose River, Adirondack Park, N.Y. Biogeochemistry 3: 121–128.
Driscoll, C. T., C. P. Yatsko, and F. J. Unangst. 1987. Longitudinal and temporal trends in the water chemistry of the North Branch of the Moose River. Biochemistry 3: 37–61.
Driscoll, C. T., and R.M. Newton. 1985. Chemical characteristics of Adirondack lakes. Environmental Science and Technology 19: 1018–1024.
Galloway, J. N., C. L. Schofield, G. R. Hendrey, E. R. Altwicker, and D. E. Troutman. 1980. An analysis of lake acidification using annual budgets.in D. Drablos and A. Tollan (eds.). Ecological impact of acid precipitation — proceedings of an international conference. Oslo, Norway, SNCF Project: 254–255.
Gran, G. 1952. Determination of the equivalence point in potentiometric titrations — part II. The analyst 77: 661–671.
Gherini, S. A., L. Mok, R. J. M. Hudson, G. F. Davis, C. W. Chen, and R. A. Goldstein. 1985. The ILWAS model: formulation and application. Water, Air and Soil Pollution 26: 425–459.
Goldstein, R. A., S. A. Gherini, C. W. Chen, L. Mok, and R. J. M. Hudson. 1984. Integrated Lake-Watershed Acidification Study (ILWAS): a mechanistic ecosystem analysis. Philosophical Transactions of the Royal Society of London B, 305: 409–425.
Harstad, L. E. 1983. Geologic controls on the sensitivity of Woodruff Pond, Newcomb, New York, to acidification. Smith College, Department of Geology, Northampton, Mass., B. S. honors thesis.
Hemond, H. F., and K. N. Eshleman. 1984. Neutralization of acid deposition by nitrate retention of Bickford Watershed, Massachusetts. Water Resources Research 20: 1718–1724.
Johannes, A. H., E. R. Altwicker, and N. L. Cleseri. 1981 Characterization of acidic precipitation in the Adirondack Region. Palo Alto, California, Electric Power Research Institute, Report EA-1826.
Johnson, N. M., G. E. Likens, F. H. Bormann, D. W. Fisher, and R. S. Pierce. 1969. A working model for the variation in stream water chemistry at the Hubbard Brood Experimental Forest, New Hampshire. Water Resources Research 5: 1353–1363.
Lane, E. W., and K. Lei. 1950. Streamflow variability. American Society Civil Engineers Transaction 115: 1084–1134.
Murdoch, P. S., N. E. Peters, and R. M. Newton. Hydrologic analysis of two headwater lake basins of differing lake pH in the west-central Adirondack Mountains, New York. U.S. Geological Survey Water Resources Investigations 84-4313. In Press.
Newton, R. M., Jill Weintraub, and R. H. April. 1987. The relationship between surface-water chemistry and geology of the North Branch of the Moose River. Biogeochemistry. 3: 21–35.
Peters, N. E., and P. S. Murdoch. 1985. Hydrogeologic comparison of an acidic-lake basin with a neutral-lake basin in the west-central Adirondack mountains, New York. Water, Air and Soil Pollution 26: 387–402.
Pfeiffer, M. H. and P. J. Festa. 1980. Acidity status of lakes in the Adirondack region of New York in relation to fish resources. Albany, New York State Department of Environmental Conversation, 1 v.
Pinder, G. F., and J. F. Jones. 1969. Determination of the ground-water component of peak discharge from the chemistry of total runoff. Water Resources Research 5: 438–445.
Rantz, S. E. 1982. Measurement and computation of streamflow — volume 1, measurement of stage and discharge. U.S. Geological Survey Water-Supply Paper 2175.
Rascher, C. M., C. T. Driscoll, and N. E. Peters. 1987. Concentration and flux of solutes from snow and forest floor during snowmelt in the West-central Adirondack Mountains, New York. Biogeochemistry. 3: 209–224.
Raynal, D. J., F. S. Raleigh, and A. J. Molliter. 1983. Characterization of Atmospheric Deposition and Ionic Input at Huntington Forest, Adirondack Mountains, New York. Syracuse, State University of New York, college of Environmental Science and Forestry, Report ESF 83-003.
Schofield, C. L. 1977. Acidification of Adirondack lakes by atmospheric precipitation — extent and magnitude of the problem. Albany, New York, State Department of Environmental Conversation, 1 v.
Searcy, J. K. 1959. Flow-duration curves — Manual of hydrology, Part 2, Lowflow techniques. U.S. geological Survey Water-Supply Paper 1542-A.
Slavine, W. 1968. Atomic absorption spectoscopy. New York, Wiley-Interscience.
Till, Roger. 1974. Statistical methods for the earth scientist—an introduction. New York, John Wiley.
Troutman, D. E., and N. E. Peters. 1982. Deposition and transport of heavy metals in three lake basins affected by acid precipitation in the Adirondack Mountains, New York. In: L. H. Keith (ed.). Energy and Environmental Chemistry, Vol. 2. Acid rain. Washington, D.C., American Chemical Society: 33–61.
Van Breeman, N., J. Mulder, and C. T. Driscoll. 1983. Acidification and alkalization of soils. Plant and Soil 75: 283–308.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peters, N.E., Driscoll, C.T. Hydrogeologic controls of surface-water chemistry in the Adirondack region of New York State. Biogeochemistry 3, 163–180 (1987). https://doi.org/10.1007/BF02185191
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02185191