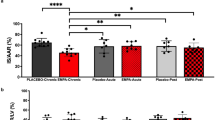
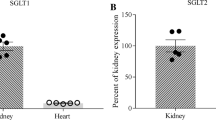
Summary
Metabolic acidosis was produced in two groups of isolated, glucose-perfused beating rat hearts. The first group (control) was untreated whereas the second group was pretreated for 48 h by the addition of verapamil (1.2 g/L) to the drinking water. Untreated hearts all developed asystole during a 30 min perfusion with an acidotic substrate (pH=6.8) or during subsequent reequilibration with physiologic substrate (pH=7.4). Prior to asystole, all untreated hearts showed evidence of severe mechanical and biochemical deterioration evaluated by31P NMR spectroscopy. In contrast, hearts of treated rats showed less mechanical and metabolic deterioration, and all recovered during reequilibration. The mechanism of protection of verapamil against the effects of metabolic acidosis is unclear but appears to be related to preserved mitochondrial function by the drug and not to a reduced demand for energy.
Similar content being viewed by others
References
Spitzer KW Hogan PM. The effects of acidosis and bicarbonate on action potential repolarization in canine cardiac Purkinje fibers.J Gen Physiol 1979;73:199–218.
Piwnica-Worms D, Jacob R, Horres CR, Lieberman M. Na/H exchange in chick heart cells.J Gen Physiol 1985;85:43–64.
Weiss J, Couper GS, Hiltbrand B, Shine KI. Role of acidosis in early contractile dysfunction during ischemia: evidence from pH measurements.Am J Physiol 1984;247:(Heart and Circ Physiol 16) H760-H767.
Kohlhardt, Haap K, Figulla HR. Influence of low extracellular pH upon the Ca inward current and isometric contractile force in mammalian ventricular myocardium.Pflueg Arch 1976;366:31–38.
Steenbergen C, Deleeuw G, Rich T, Williamson JR. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart.Circ Res 1977;41:849–58.
Wikman-Coffelt J, Sievers R, Parmley WW, Jasmin G. Cardiomyopathic and healthy hamster hearts: mitochondrial activity may regulate cardiac performance.Cardiovasc Res 1986;20:471–81.
Vaughan-Jones RD, Lederer WJ, Eisner DA. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle.Nature 1983;301:522–24.
Ganong WF. Regulation of extracellular fluid composition and volume. In:Review of medical physiology 6th edition. Los Altos, CA: Lange Medical Publications 1973:536–42.
Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating concentrations of sodium and internal pH.J Mol Cell Cardiol 1985;17:1029–42.
Balasubramanian V, McNamara DB, Singh JN, Dhalla NS. Biochemical basis of heart function. X. Reduction in Na+ -K+ -stimulated ATPase activity in failing rat heart due to hypoxia.Can J Physiol Pharmacol 1973;51:504–10.
Mechmann S, Pott L. Identification of Na-Ca exchange current in single cardiac myocytes.Nature 1985;319(6054):597–99.
Hochachka PW, Mommsen TP. Protons and anaerobiosis.Science 1983;219:1391–97.
Mansour RE. Multiple forms of heart phosphofructokinase. In: Chance B, Estabrook RW, Williams JR, eds.Control of energy metabolism. New York:Academic Press, 1965:81–87.
Kentish JC. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle.J Physiol 1986;370:585–604.
Fleckenstein A.Calcium antagonism in heart and smooth muscle. New York: J Wiley and Sons, 1983.
Nayler WG. Calcium antagonists. In: Shibata S, Bailey LE, eds.Recent developments in cardiac muscle pharmacology. Tokyo, New York: Igaku-Shoin, 1982:99–124.
Katz AM. Basic cellular mechanisms of action of the calcium-channel blockers.Am J Cardiol 1985;55:2B-9B.
Nayler WG, Grau A, Slade A. A protective effect of verapamil on hypoxic heart muscle.Cardiovasc Res 1976;10:650–62.
Nayler WG, Ferrari R, Williams A. Protective effect of pretreatment with verapamil, nifedipine and propranolol in mitrochodrial function in the ischemic and reperfused myocardium.Am J Cardiol 1980;46:242–48.
Hamm CW, Opie LH. Protection of infarcting myocardium by slow channel inhibitors.Circ Res 1983;52(suppl I):129–138.
Bourdillon PD, Poole-Wilson PA. The effects of verapamil, quiescence, and cardioplegia on calcium exchange and mechanical function in ischemic rabbit myocardium.Circ Res 1982;50:360–68.
Sievers R, Parmley WW, James T, Wikman-Coffelt J. Energy levels at systole vs diastole in normal hamster hearts vs myopathic hamsters hearts.Circ Res 1983;53:759–66.
Wikman-Coffelt J, Coffelt RJ. Flexible tube: counter current heat exchanger.Res Sci Instrum 1985;56:165–68.
Wikman-Coffelt LF, Coffelt RJ, Rouleau JL, et al. A new apex-ejecting perfused rat heat preparation.Cardiovasc Res 1983;17:747–55.
Wikman-Coffelt J, Sievers R, Coffelt RJ, Parmley WW. The cardiac cycle: regulation and energy oscillations.Am J Physiol 1983;245:H359-H362.
James TL. In vivo nuclear magnetic resonance spectroscopy. In: Moss AA, Ring EJ, Higgins CB, eds. NMR, CT and Interventional Radiology. San Francisco, Radiology, Research and Education Foundation 1984:235–44.
Chance B, Cleff S, Leigh JS, Jr., et al. Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: a gated 31-P NMR study.Proc Natl Soc Sci 1981;78:6714–18.
Opie L. Effect of extracellular pH on function and metabolism of isolated perfused rat heart.Am J Physiol 1965;109:(6):1075–80.
Smith HJ, Briscoe MG. The relative sensitization by acidosis of five calcium blockers in cat papillary muscle.J Mol Cell Cardiol 1985;17:709–16.
Watson RM, Markle DR, McGuire DA, et al. Effect of verapamil on pH of ischemic canine myocardium.J Am Coll Cardiol 1985;5:1345–54.
Couper GS, Weiss J, Hiltbrand B, Shine KI. Extracellular pH and tension during ischemia in the isolated rabbit ventricle.Am J Physiol 1984;267: H916-H927.
Wikman-Coffelt J, Sievers R, Parmley WW, Jasmin G. Verapamil preserves adenine nucleotide pool in cardiomyopathic Syrian hamster.Am J Physiol 1986;250:H22-H28.
Rouleau J-L, Chatterjee K, Ports TA, et al. Mechanism of relief of pacing-induced angina with oral verapamil: reduced oxygen demand.Circulation 1983;67:94–100.
Davenport N, Goldstein RE, Bolli R, Epstein SE. Blood flow in infarct and surviving myocardium: implications regarding the action of verapamil on the acutely ischemic dog heat.J Am Coll Cardiol 1984;4:956–65.
Cheung JY, Leaf A, Bonventre JV. Mechanism of protection by verapamil and nifedipine from anoxic injury in isolated cardiac myocytes.Am J Physiol 1984;246:C323-C329.
Watts JA, Maiorano LF, Maiorano PC. Protection by verapamil of globally ischemic rat hearts: energy preservation, a partial explanation.J Mol Cell Cardiol 1985;17:797–804.
Daly MJ, Elz JS, Nayler WG. The effect of verapamil on ischemia-induced changes to the sarcolemma.J Mol Cell Cardiol 1985;17:667–74.
Lange R, Ingwall J, Hale SL, et al. Preservation of high-energy phosphate by verapamil in reperfused myocardium.Circulation 1984;70:734–41.
Author information
Authors and Affiliations
Additional information
Supported in part by the George P. Smith Foundation and the Susan and Don Schleicher Fund.
Rights and permissions
About this article
Cite this article
Markiewicz, W., Wu, S.S., Sievers, R. et al. Beneficial effects of verapamil during metabolic acidosis in isolated perfused rat hearts. Cardiovasc Drug Ther 1, 493–502 (1988). https://doi.org/10.1007/BF02125732
Issue Date:
DOI: https://doi.org/10.1007/BF02125732