Summary
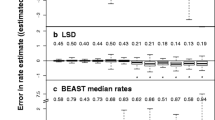
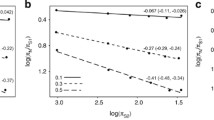
Based on mitochondrial DNA (mt-DNA) sequence data from a wide range of primate species, branching order in the evolution of primates was inferred by the maximum likelihood method of Felsenstein without assuming rate constancy among lineages. Bootstrap probabilities for being the maximum likelihood tree topology among alternatives were estimated without performing a maximum likelihood estimation for each resampled data set. Variation in the evolutionary rate among lineages was examined for the maximum likelihood tree by a method developed by Kishino and Hasegawa. From these analyses it appears that the transition rate of mtDNA evolution in the lemur has been extremely low, only about 1/10 that in other primate lines, whereas the transversion rate does not differ significantly from that of other primates. Furthermore, the transition rate in catarrhines, except the gibbon, is higher than those in the tarsier and in platyrrhines, and the transition rate in the gibbon is lower than those in other catarrhines. Branching dates in primate evolution were estimated by a molecular clock analysis of mtDNA, taking into account the rate of variation among different lines, and the results were compared with those estimated from nuclear DNA. Under the most likely model, where the evolutionary rate of mtDNA has been unifrom within a great apes/human calde, human/chimpanzee clustering is preferred to the alternative branching orders among human, chimpanzee, and gorilla.
Similar content being viewed by others
References
Aiello LC (1986) The relationships of the Tarsiiformes: a review of the case for Haplorhini. In: Wood B, Martin L, Andrews P (eds) Major topics in primate and human evolution. Cambridge University Press, Cambridge, pp 47–65
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Contr AC-19:716–723
Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith ALH, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–464
Anderson S, Bruijn MHL, Coulson AR, Eperon IC, Sanger F, Young IG (1982) The complete sequence of bovine mitochondrial DNA: conserved features of the mammalian mitochondrial genome. J Mol Biol 156:683–717
Andrews P (1986) Fossil evidence on human origins and dispersal. Cold Spring Harbor Symp Quant Biol 52:419–428
Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA (1981) Sequence and gene organization of mouse mitochondrial DNA. Cell 26:167–180
Bonner TI, Heinemann R, Todaro GJ (1980) Evolution of DNA sequences has been retarded in Malagasy primates. Nature 286:420–423
Britten RJ (1986) Rates of DNA sequence evolution differ between taxonomic groups. Science 231:1393–1398
Brown WM, Prager EM, Wang A, Wilson AC (1982) Mitochondrial DNA sequence of primates: tempo and mode of evolution. J Mol Evol 18:225–239
Caccone A, Powell JR (1989) DNA divergence among hominoids. Evolution 43:925–942
de Jong WW, Goodman M (1988) Anthropoid affinities ofTarsius supported by lens αA-crystallin sequences. J Hum Evol 17:575–582
Delson E (1980) Fossil macaques, phyletic relationships and a scenario of deployment. In: Lindburg DG (ed) The macaques: studies in ecology, behavior and evolution. Van Nostrand-Reinhold, New York, pp 10–30
Farris JS (1972) Estimating phylogenetic trees from distance matrices. Am Nat 106:645–668
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Felsenstein J (1988) Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet 22:521–565
Fitch WM, Markowitz E (1970) An improved method for determining codon variability in a gene and its application to the rate of fixations of mutations in evolution. Biochem Genet 4:579–593
Fitch DHA, Mainone C, Slightom JL, Goodman M (1988) The spider monkey ψη-globin gene surrounding sequences: recent or ancient insertions of LINEs and SINEs? Genomics 3:237–255
Fooden J (1980) Classification and distribution of living macaques (Macaca lecépède). In: Lindburg DG (ed) The macaques: studies in ecology, behavior and evolution. Van Nostrand-Reinhold, New York, pp 1–9
Gebo DL (1986) Anthropoid origins—the foot evidence. J Hum Evol 15:421–430
Gingerich PD (1981) Early Cenozoic Omomyidae and the evolutionary history of tarsiiform primates. J Hum Evol 10:345–374
Gingerich PD (1986) Temporal scaling of molecular evolution in primates and other mammals. Mol Biol Evol 3:205–221
Hasegawa M, Kishino H (1989a) Heterogeneity of tempo and mode of mitochondrial DNA evolution among mammalian orders. Jpn J Genet 64:243–258
Hasegawa M, Kishino H (1989b) Confidence limits on the maximum-likelihood estimate of the hominoid tree from mitochondrial-DNA sequences. Evolution 43:672–677
Hasegawa M, Yano T (1984) Maximum likelihood method of phylogenetic inference from DNA sequence data. Bull Biomet Soc Jpn 5:1–7
Hasegawa M, Kishino H, Yano T (1985) Dating of the humanape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Hasegawa M, Kishino H, Yano T (1987) Man's place in Hominoidea as inferred from molecular clocks of DNA. J Mol Evol 26:132–147
Hasegawa M, Kishino H, Yano T (1989) Estimation of branching dates among primates by molecular clocks of nuclear DNA which slowed down in Hominoidea. J Hum Evol 18:461–476
Hayasaka K, Gojobori T, Horai S (1988) Molecular phylogeny and evolution of primate mitochondrial DNA. Mol Biol Evol 5:626–644
Kikuno R, Hayashida H, Miyata T (1985) Rapid rate of rodent evolution. Proc Jpn Acad B 61:153–156
Kimura M (1983) The neutral theory of molecular evolution. Cambridge University Press, Cambridge
Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J Mol Evol 29:170–179
Kishino H, Hasegawa M (1990) Converting distance to time: an application to human evolution. Methods Enzymol 183:550–570
Kishino H, Miyata T, Hasegawa M (1990) Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. J Mol Evol 31:151–160
Koop BF, Goodman M, Xu P, Chan K, Slightom JL (1986) Primate η-globin DNA sequences and man's place among the great apes. Nature 319:234–238
Li W-H, Tanimura M (1987) The molecular clock runs more slowly in man than in apes and monkeys. Nature 326:93–96
Maeda N, Bliska JB, Smithies O (1983) Recombination and balanced chromosome polymorphism suggested by DNA sequences 5′ to the human δ-globin gene. Proc Natl Acad Sci USA 80:5012–5016
Maeda N, Wu C-I, Bliska J, Reneke J (1988) Molecular evolution of intergenic DNA in higher primates: pattern of DNA changes, molecular clock, and evolution of repetitive sequences. Mol Biol Evol 5:1–20
Miyamoto MM, Slightom JL, Goodman M (1987) Phylogenetic relations of humans and African apes from DNA sequences in the ψη-globin region. Science 238:369–373
Miyamoto MM, Koop BF, Slightom JL, Goodman M (1988) Molecular systematics of higher primates: genealogical relations and classification. Proc Natl Acad Sci USA 85:7626–7631
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Pilbeam D (1985) Patterns of hominoid evolution. In: Delson E (ed) Ancestors: the hard evidence. Alan R Liss, New York, pp 51–59
Pocock RI (1918) On the external characters of lemurs and ofTarsius. Proc Zool Soc Lond 1918:19–53
Rabinowitz M, Swift H (1970) Mitochondrial nucleic acids and their relation to the biogenesis of mitochondria. Physiol Rev 50:376–427
Rosenberger AL, Szalay FS (1980) On the tarsiiform origins of Anthropoidea. In: Ciochon RL, Chiarelli AB (eds) Evolutionary biology of the New World monkeys and continental drift. Plenum Press, New York, pp 139–157
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakamoto Y, Ishiguro M, Kitagawa G (1986) Akaike information criterion statistics. Reidel, Dordrecht
Sarich VM, Wilson AC (1967) Immunological time scale for hominid evolution. Science 158:1200–1203
Schwartz JH, Tattersall I (1987) Tarsiers, adapids and the interity of Strepsirhini. J Hum Evol 16:23–40
Sibley CG, Ahlquist JE (1984) The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol 20:2–15
Sibley CG, Ahlquist JE (1987) DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol 26:99–121
Simons EL (1972) Primate evolution: an introduction to man's place in nature. Macmillan, New York
Simpson GG (1945) The principles of classification and a classification of mammals. Bull Am Mus Nat Hist 85:1–350
Sneath PHA, Sokal RR (1973) Numerical taxonomy: the principle and pratice of numerical classification. WH Freeman, San Francisco
Vawter L, Brown WM (1986) Nuclear and mitochondrial DNA comparisons reveal extreme rate variation in the molecular clock. Science 234:194–196
Wilson AC, Carlson SS, White TJ (1977) Biochemical evolution. Annu Rev Biochem 46:573–639
Wu C-I, Li W-H (1985) Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci USA 82:1741–1745
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasegawa, M., Kishino, H., Hayasaka, K. et al. Mitochondrial DNA evolution in primates: Transition rate has been extremely low in the lemur. J Mol Evol 31, 113–121 (1990). https://doi.org/10.1007/BF02109480
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02109480